35
Chemotherapy of Malaria
This chapter will be most useful after having a basic understanding of the material in Chapter 49, Chemotherapy of Malaria in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition. In addition to the material presented here, Chapter 49 in the 12th Edition contains:
• Tables 49-2, 49-3, and 49-4 which provide information about appropriate regimens, including adult and pediatric dosages, for the prevention, treatment, and presumptive self-treatment of malaria
• Figure 49-4 which is an algorithm approach to the treatment of malaria
LEARNING OBJECTIVES
 Know the stages of the malaria parasite in the human body.
Know the stages of the malaria parasite in the human body.
 Classify antimalarial drugs into those that are effective against only the blood stages of the parasite, those that are effective against both the blood and liver stages, and those that are effective against only the liver stages of the parasite.
Classify antimalarial drugs into those that are effective against only the blood stages of the parasite, those that are effective against both the blood and liver stages, and those that are effective against only the liver stages of the parasite.
 Understand the use of antimalarial drugs in clinical context, particularly with regard to their mechanism of action, therapeutic uses, and toxicities.
Understand the use of antimalarial drugs in clinical context, particularly with regard to their mechanism of action, therapeutic uses, and toxicities.
 Describe the principles and guidelines for the chemoprophylaxis and treatment of malaria.
Describe the principles and guidelines for the chemoprophylaxis and treatment of malaria.
DRUGS INCLUDED IN THIS CHAPTER
Artemisinin and Artemisinin—combination therapies
Atovaquone/Proguanil (MALARONE)
Chloroquine (ARALEN)
Doxycycline—see Chapter 41
Hydroxychloroquine (PLAQUENIL)
Mefloquine (LARIUM)
Primaquine
Pyrimethamine (DARAPRIM)
Quinine/Quinidine
Sulfadoxine/Pyrimethamine (FANSIDAR)
CRITERIA FOR THE DIAGNOSIS OF MALARIA
• Signs and symptoms consistent with malaria, including fever, chills, malaise, myalgia, headache, ± severe malaria criteria (see Table CRITERIA FOR DIAGNOSIS OF SEVERE MALARIA), plus
• Demonstration of the malaria parasite:
▶ Light microscope examination of stained thin or thick smear
▶ Rapid diagnostic test
▶ Molecular or biophysical-based testing
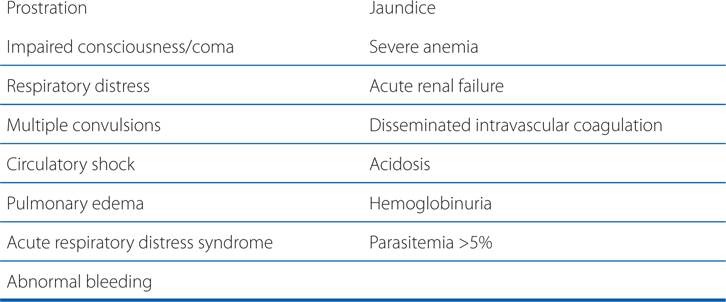
CRITERIA FOR DIAGNOSIS OF SEVERE MALARIA
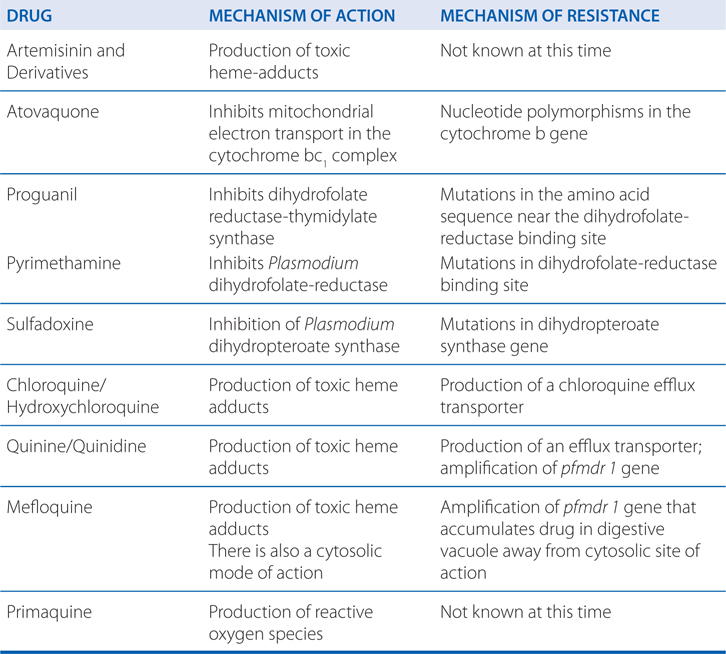
ANTIMALARIAL DRUG MECHANISMS OF ACTION AND RESISTANCE
CURRENT ARTEMISININ COMBINATION THERAPY (ACT) REGIMENS
Artemether-lumefantrine*
Artesunate-mefloquine
Artesunate-amodiaquine
Artesunate-sulfoxadine-pyrimethamine
Dihydroartemisinin-piperaquine
*Lumefantrine shares structural similarities with mefloquine and is only formulated with artemether (COARTEM)
A 43-year-old woman develops the signs and symptoms of uncomplicated malaria caused by Plasmodium falciparum. Her symptoms include fever, chills, malaise, and headache. A microscopic examination of a blood smear confirms the diagnosis. She is treated with the combination therapy artemether-lumefantrine.
a. Why is she treated with the combination product rather than either artemether or lumefantrine alone?
Artemether-lumefantrine is one of several artemisinin-based combination therapies (ACTs). The artemisinins are very potent and fast-acting antimalarials. However, the emergence of P. falciparum isolates with an increased tolerance to artemisinins has recently been found. The primary goal of these combination products is to increase treatment efficacy and reduce selection pressure for the emergence of drug resistance. The short t½ of the artemisinins results in substantial treatment failure rates when artemisinins are used as monotherapy. Combining an artemisinin with a longer-lasting partner drug assures sustained antimalarial activity.
b. What are the pharmacokinetic properties of lumefantrine that make it a good choice in combination with artemether?
Artemisinins rapidly achieve peak serum concentrations; intramuscular artemether peaks at 2 to 6 hours, due to a depot effect at the injection site. Lumefantrine has a large apparent volume of distribution and a terminal elimination t½ of 4 to 5 days. Administration with a high-fat meal is recommended because it significantly increases lumefantrine absorption. The advantages and disadvantages of the other combination drugs are discussed in detail in Chapter 49 in Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition.
A 23-year-old female college student is planning a trip to an area of Africa that is endemic for chloroquine-resistant P. falciparum. She has been advised to take atovaquone-proguanil for chemoprophylaxis.
a. When should the drug be started?
Chemoprophylaxis for malaria should begin before exposure and preferably before the traveler leaves home. This is to establish therapeutic blood concentrations and to detect early signs or symptoms of intolerance so that the regimen can be modified before departing. The regimen should be taken daily while in the malarious area and for 7 days after leaving the area. (see Table 35-1 below and Table 49-2 in Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition for detailed chemoprophylactic regimens.)
TABLE 35-1 Regimens for the Prevention of Malaria in Nonimmune Individuals (For complete details see Table 49-2 in Goodman & Gilman’s The Pharmacological Basis of Therapeutics, 12th Edition, Chapter 49)
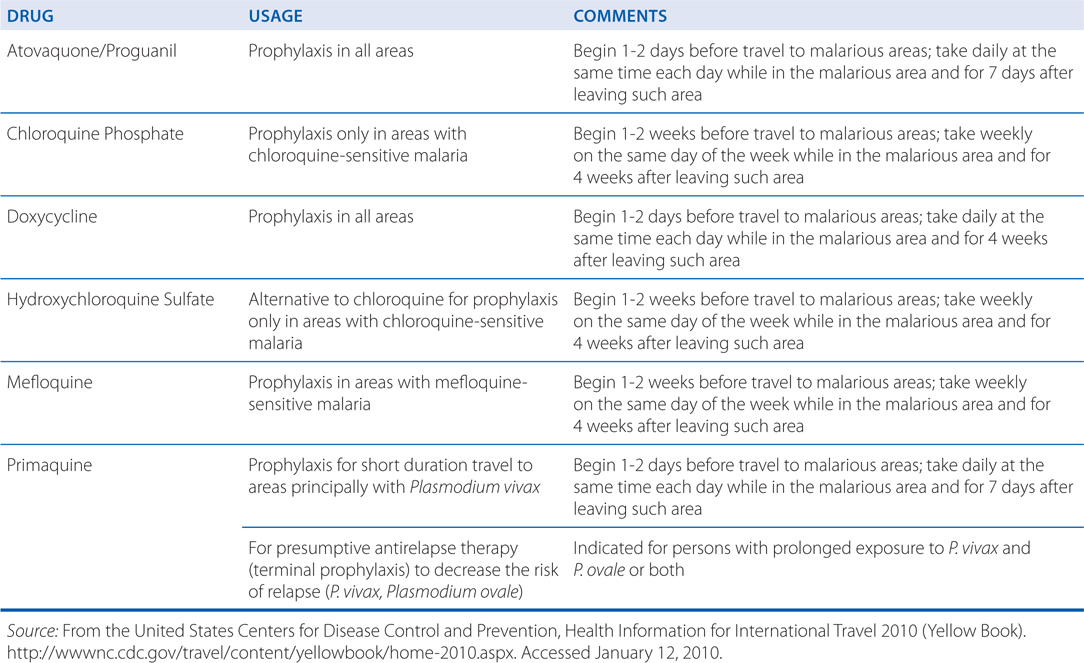
b. What side effects might this patient expect?
This combination is generally considered safe with no serious side effects. The most common side effects are nausea, vomiting, diarrhea, and headache.
c. Why is the combination atovaquone-proguanil used rather than either drug alone?
Resistance to atovaquone when used alone occurs easily. The addition of proguanil markedly reduces the frequency of atovaquone resistance. However, once atovaquone resistance is present the effect of adding the proguanil diminishes.
d. How do the mechanisms of atovaquone and proguanil work synergistically?
Atovaquone acts selectively on the mitochondrial cytochrome bc1 complex to inhibit electron transport and collapse the mitochondrial membrane potential. The antimalarial activity of proguanil is ascribed to cycloguanil (structurally similar to pyrimethamine), a selective inhibitor of the bifunctional plasmodial dihydrofolate reductase-thymidylate synthetase that is crucial for parasite purine and pyrimidine synthesis. Inhibition of this enzyme causes inhibition of DNA synthesis and depletion of folate cofactors. The synergy between proguanil and atovaquone results from the ability of proguanil to enhance the mitochondrial toxicity of atovaquone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


