C. It is predictable that clinical manifestations of toxicity caused by chemotherapeutic drugs often include myelosuppression (leukopenia, thrombocytopenia, or anemia), nausea, vomiting, diarrhea, mucosal ulceration, dermatitis, and alopecia as these represent activity at normal rapidly dividing cells. Myelosuppression is the dose-limiting factor for many chemotherapeutic drugs and is the most common toxicity that leads to temporary or permanent withdrawal of therapy (usually reversible with discontinuation of the chemotherapeutic agent).
II. Drug Resistance. Resistance to chemotherapeutic drugs often occurs and has many causes (some chemotherapy agents lead to induction of drug-metabolizing enzymes; many solid tumors grow so rapidly that portions of the tumor are poorly vascularized, preventing therapeutic concentrations from reaching many target cells and mutations in the drug-binding domain of the target enzyme).
III. Classification (Table 42-1)
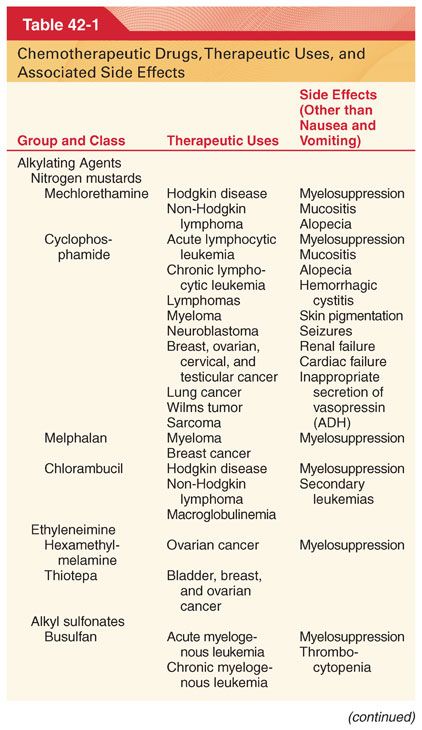
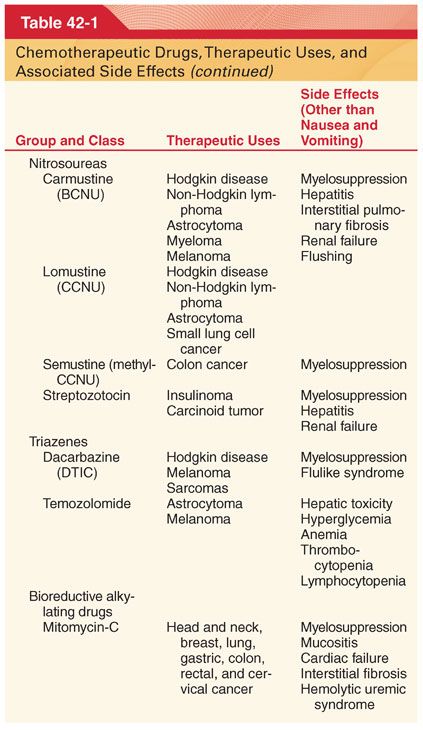
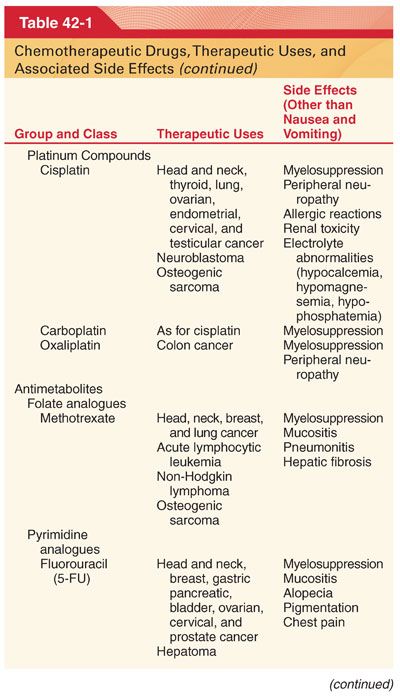
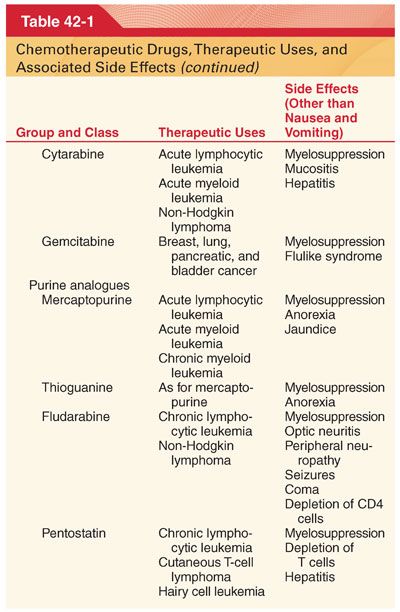
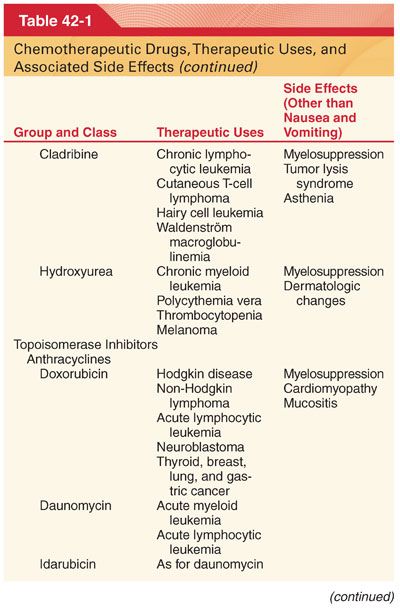
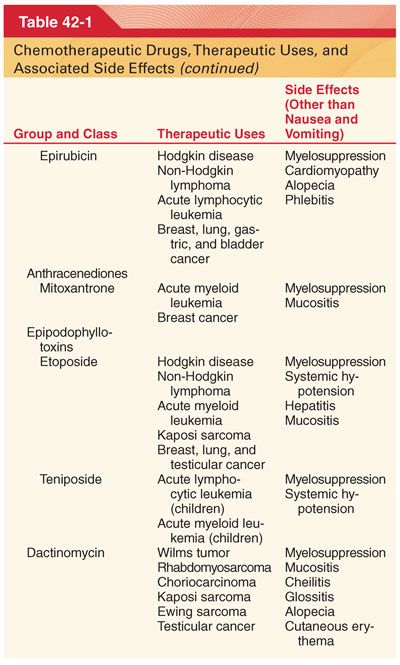

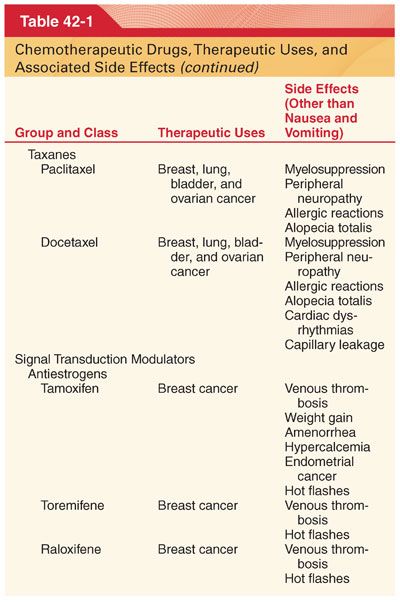
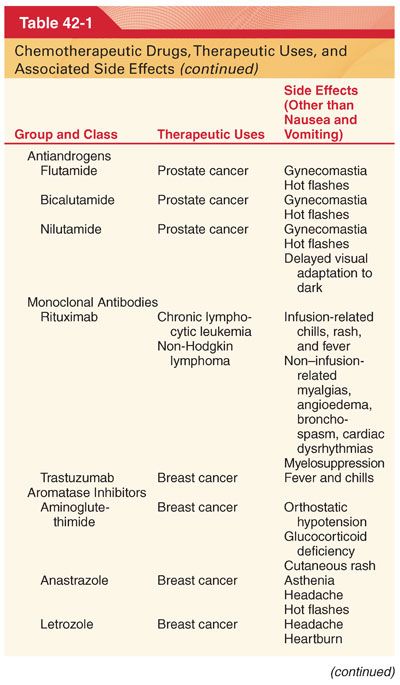

A. Knowledge of drug-induced adverse effects and evaluation of appropriate laboratory tests (hemoglobin, platelet count, white blood cell count, coagulation profile, arterial blood gases, blood glucose, plasma electrolytes, liver and renal function tests, electrocardiogram [ECG], and radiograph of the chest) are useful in the preoperative evaluation of patients being treated with specific chemotherapeutic drugs.
B. Immunosuppression makes these patients susceptible to iatrogenic infections, thus asepsis and the use of appropriate prophylactic antibiotics is critical.
C. A history of severe vomiting or diarrhea may be associated with electrolyte disturbances and decreased intravascular fluid volume.
D. The existence of mucositis makes placement of pharyngeal airways, laryngeal mask airways, and esophageal catheters questionable.
E. Theoretically, the effects of succinylcholine may be prolonged if plasma cholinesterase activity is decreased by chemotherapeutic drugs.
IV. Toxicities
A. Chemotherapeutic drugs typically target proteins or nucleic acids that are common to malignant and nonmalignant cells and thus possess a narrow therapeutic index (see Table 42-1).
B. Patients who have a history of chemotherapy-induced nausea and vomiting are not necessarily prone to postoperative nausea and vomiting. Development of serotonin antagonists as effective antiemetics in addition to combination antiemetic regimens has facilitated the tolerance of emetogenic chemotherapeutic drugs.
V. Alkylating Agents (nitrogen mustards, alkyl sulfonates, nitrosoureas, and triazenes) form covalent alkyl bonds with nucleic acid bases, resulting in intrastrand or interstrand DNA cross-links that are toxic to cells undergoing division.
A. Side Effects
1. Bone marrow suppression is the most important dose-limiting factor in the clinical use of alkylating drugs, especially busulfan. Lymphocytopenia is usually present within 24 hours. Variable degrees of depression of platelet and erythrocyte counts may occur. Hemolytic anemia is predictably present.
2. All alkylating drugs are powerful central nervous system (CNS) stimulants, manifesting most often as nausea and vomiting.
3. Pneumonitis and pulmonary fibrosis are potential adverse effects of alkylating drugs.
4. Inhibition of plasma cholinesterase activity may be present for as long as 2 to 3 weeks after administration of chemotherapy regimens that include an alkylating agent and can lead to prolonged skeletal muscle paralysis after administration of succinylcholine.
5. Rapid drug-induced destruction of malignant cells can produce increased purine and pyrimidine breakdown, leading to uric acid–induced nephropathy (recommend adequate fluid intake, alkalinization of the urine, and administration of allopurinol be established before drug treatment).
B. Nitrogen Mustards
1. Mechlorethamine is a rapidly acting nitrogen mustard administered intravenously (IV) to minimize local tissue irritation (intensely powerful vesicants requiring that gloves be worn by personnel handling the drug).
a. Clinical Uses. Mechlorethamine produces beneficial effects in the treatment of Hodgkin disease and, less predictably, in other lymphomas.
b. Side Effects. The major side effects of mechlorethamine include nausea, vomiting, and myelosuppression (leukopenia and thrombocytopenia constitute the principal limitation on the amount of drug that can be given). Latent viral infections may be unmasked by treatment with mechlorethamine. Thrombophlebitis is a potential complication, and extravasation of the drug results in severe local tissue reactions.
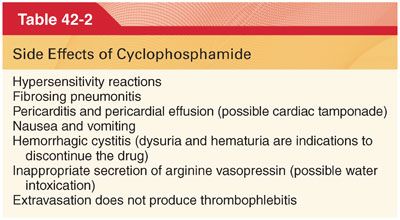
2. Cyclophosphamide is absorbed after oral or parenteral administration and is subsequently activated in the liver to aldophosphamide for transport to target tissues. Target cells are able to convert aldophosphamide to highly cytotoxic metabolites than alkylate DNA.
a. Clinical Uses. Cyclophosphamide is used in the treatment of a wide range of cancers (combination with methotrexate and fluorouracil as adjuvant therapy after surgery for breast cancer) and inflammatory diseases.
b. Side Effects (Table 42-2)
3. Melphalan is a phenylalanine derivative of nitrogen mustard with a range of activity similar to other alkylating drugs. It is not a vesicant.
a. Side effects of melphalan are primarily hematologic and are similar to those of other alkylating drugs. It is usually necessary to maintain a significant degree of bone marrow depression (leukocyte count 3,000 to 5,000 cells/mm3) to achieve optimal therapeutic effects. Pulmonary fibrosis is possible. Nausea and vomiting are not common side effects of melphalan. Alopecia does not occur.
4. Chlorambucil is the treatment of choice in chronic lymphocytic leukemia and in primary (Waldenström) macroglobulinemia. A marked increase in the incidence of leukemia and other tumors has been noted with the use of this drug for the treatment of polycythemia vera.
a. Side Effects. Cytotoxic effects of chlorambucil on the bone marrow, lymphoid organs, and epithelial tissues are similar to those observed with other alkylating drugs. Its myelosuppressive action is usually moderate, gradual, and rapidly reversible. Pulmonary fibrosis is possible. Nausea and vomiting are frequent.
C. Alkyl Sulfonates
1. Busulfan produces remissions in up to 90% of patients with chronic myelogenous leukemia. The drug is of no value in the treatment of acute leukemia.
a. Side Effects. Busulfan can produce progressive pulmonary fibrosis in up to 4% of patients (prognosis after appearance of clinical symptoms is poor, with a median survival of 5 months). Myelosuppression and thrombocytopenia are important side effects. Hyperuricemia resulting from extensive purine catabolism is possible (allopurinol is recommended to minimize renal complications).
D. Nitrosoureas (carmustine, lomustine, semustine, streptozocin) possess a wide spectrum of activity for human malignancies including intracranial tumors, melanomas, and gastrointestinal and hematologic malignancies (high lipid solubility results in passage across the blood–brain barrier and efficacy in the treatment of meningeal leukemias and brain tumors). With the exception of streptozocin, the clinical use of nitrosoureas is limited by profound drug-induced myelosuppression.
1. Carmustine is capable of crossing the blood–brain barrier and is used to treat meningeal leukemia and primary as well as metastatic brain tumors.
a. Side Effects. Carmustine has been associated with interstitial pneumonitis and fibrosis much like bleomycin. The incidence of pulmonary toxicity is in the range of 20% to 30% (mortality in those affected of 24% to 90%). The cumulative dose is the major risk factor. A unique side effect of carmustine is a delayed onset (after approximately 6 weeks of treatment) of leukopenia and thrombocytopenia.
2. Lomustine and Semustine. Lomustine and its methylated analogue semustine possess similar clinical toxicity to carmustine. Lomustine appears to be more effective than carmustine in the treatment of Hodgkin disease.
3. Streptozocin has a unique affinity for β cells of the islets of Langerhans and has proved useful in the treatment of human pancreatic islet cell carcinoma and malignant carcinoid.
a. Side Effects. Approximately 70% of patients receiving this drug develop hepatic or renal toxicity. Hyperglycemia can occur as a result of selective destruction of pancreatic β cells. Myelosuppression is not produced by this drug.
4. Mitomycin is of value in the palliative treatment of gastric adenocarcinoma in combination with fluorouracil and doxorubicin.
a. Side Effects. Myelosuppression is a prominent side effect of mitomycin and is characterized by severe leukopenia and thrombocytopenia, which may be delayed in appearance. Mitomycin is capable of inducing pulmonary fibrosis, with an incidence ranging between 3% and 12%. Glomerular damage resulting in renal failure is a rare but well-recognized complication.
VI. Platinating Drugs
A. Cisplatin results in chemotherapeutic effects resembling DNA alkylating drugs by cross-linking adjacent or opposing guanine bases to disrupt DNA. The drug must be administered IV because oral ingestion is ineffective. High concentrations of cisplatin are found in the kidneys, liver, intestines, and testes, but there is poor penetration into the CNS (treatment of many nonhematologic malignancies, including lung, bladder, testicular, and ovarian cancer).
1. Side Effects
a. Renal toxicity is prominent and becomes the dose-limiting toxic effect of cisplatin.
b. Hypomagnesemia that is associated with cisplatin’s renal tubular injury may predispose to cardiac dysrhythmias and decrease the dose requirements for neuromuscular blocking drugs.
c. Ototoxicity caused by cisplatin is manifested by tinnitus and hearing loss in the high-frequency range.
d. Cisplatin is considered highly emetogenic, with marked nausea and vomiting occurring in almost all patients who do not receive antiemetics.
e. Peripheral sensory neuropathies, paresthesias, and loss of vibratory and position sense are common findings. Most neuropathies are reversible, although symptoms may persist for months.
VII. Antimetabolites (folate analogues, pyrimidine analogues, purine analogues) are particularly effective in destroying cells during the S phase of the cell cycle, which is when DNA is synthesized. Side effects (myelosuppression and mucositis) reflect effects on proliferating but nonmalignant cells.
A. Folate Analogues
1. Methotrexate is a poorly lipid-soluble folate analogue that is effective in the treatment of different hematologic and nonhematologic cancers. Significant metabolism of methotrexate does not seem to occur, with more than 50% of the drug appearing unchanged in urine.
a. Clinical Uses. Methotrexate is a useful drug in the treatment of acute lymphoblastic leukemia in children but not adults. Choriocarcinoma is effectively treated with this drug. Improvement in the clinical manifestations of psoriasis in patients reflects the effect of methotrexate on rapidly dividing epidermal cells. This drug may also be useful in the treatment of rheumatoid arthritis. Methotrexate is poorly transported across the blood–brain barrier, and neoplastic cells that have entered the CNS probably are not affected.
b. Side Effects. Leukopenia and thrombocytopenia reflect bone marrow depression. Ulcerative stomatitis and diarrhea are frequent side effects and require interruption of treatment. Hemorrhagic enteritis and death from intestinal perforation may occur. Pulmonary toxicity may take the form of fulminant noncardiogenic pulmonary edema, or a more progressive inflammation, with interstitial infiltrates and pleural effusions. Short-term or intermittent therapy with methotrexate results in increases in liver transaminase enzymes. Normal cells can be protected from lethal damage by folate antagonists with sequential administration of folinic acid (leucovorin), thymidine, or both (termed the rescue technique).
B. Pyrimidine analogues (fluorouracil and cytarabine) prevent the biosynthesis of pyrimidine nucleotides and interfere with synthesis and functioning of nucleic acids.
1. Fluorouracil lacks significant inhibitory activity on cells and must be converted enzymatically to a 5′-monophosphate nucleotide. Fluorouracil readily enters the cerebrospinal fluid.
a. Clinical Uses. Fluorouracil may be of palliative value in certain types of carcinoma, particularly of the breast and gastrointestinal tract. The drug is often used for the topical treatment of premalignant keratoses of the skin and superficial basal cell carcinomas.
b. Side effects caused by fluorouracil are difficult to anticipate because of their delayed appearance. Fluorouracil-induced myocardial ischemia is a rare cardiac toxicity that may lead to myocardial infarction up to 1 week after treatment. Myelosuppression, most frequently manifesting as leukopenia between 9 and 14 days of therapy, is a serious side effect. Thrombocytopenia and anemia may complicate treatment with fluorouracil. Loss of hair progressing to total alopecia may occur.
2. Cytarabine, like other pyrimidine antimetabolites, must be activated by conversion to the 5′-monophosphate nucleotide before inhibition of DNA synthesis can occur. Resistance to cytarabine reflects activity of cytidine deaminase, an enzyme capable of converting cytarabine to the inactive metabolite arabinosyl uracil.
a. Clinical Uses. Cytarabine is particularly useful in chemotherapy of acute granulocytic leukemia in adults.
b. Side Effects. Cytarabine is a potent myelosuppressive drug capable of producing severe leukopenia, thrombocytopenia, and anemia. Cerebellar toxicity and ataxia can occur at high doses. Thrombophlebitis at the site of infusion is common.
C. Purine Analogues (Mercaptopurine, Azathioprine, Thioguanine, Pentostatin, Cladribine)
1. Mercaptopurine is incorporated into DNA or RNA strands and either blocks further strand synthesis or causes structural alterations that damage DNA. This drug is useful in the treatment of acute leukemia in children. Allopurinol, as an inhibitor of xanthine oxidase, prevents conversion of mercaptopurine to 6-thiouric acid and thus increases the exposure of cells to mercaptopurine (dose is decreased by about one-third when the drug is combined with allopurinol).
a. Side Effects. The principal side effect of mercaptopurine is a gradual development of bone marrow depression manifesting as thrombocytopenia, granulocytopenia, or anemia several weeks after initiation of therapy. Jaundice occurs in approximately one-third of patients and is associated with bile stasis and occasional hepatic necrosis. Hyperuricemia and hyperuricosuria may occur during treatment with mercaptopurine, presumably reflecting destruction of cells (may require the use of allopurinol).
2. Thioguanine is of particular value in the treatment of acute myelogenous leukemia, especially if given with cytarabine. Minimal amounts of 6-thiouric acid are formed (may be administered concurrently with allopurinol without a decrease in dosage).
3. Pentostatin and cladribine are purine analogues that have clinical activity against a variety of indolent lymphoid tumors, with the most dramatic effects occurring in patients with hairy cell leukemia. Patients with acute leukemia and cells with high levels of adenosine deaminase activity are most likely to respond to these drugs.
4. Hydroxyurea acts on the enzyme ribonucleoside diphosphate reductase to interfere with the synthesis of DNA. The primary use of hydroxyurea is in the treatment of chronic myelogenous leukemia.
a. Side Effects. Myelosuppression manifesting as leukopenia, megaloblastic anemia, and occasionally thrombocytopenia is the major side effect produced by hydroxyurea.
VIII. Topoisomerase Inhibitors (Doxorubicin, Daunorubicin, Etoposide, Teniposide). Topoisomerases are enzymes that correct alterations in DNA, which occur during replication and transcription. Because cancer cells possess more topoisomerase activity than normal cells, there is more drug-induced DNA damage and resultant cell death. Toxicity reflects effects of inhibition of topoisomerase enzymes on normal proliferating tissues (myelosuppression, mucositis). These drugs exhibit a broad spectrum of chemotherapeutic activity being useful in the treatment of leukemia, lung, colon, and ovarian cancer.
A. Doxorubicin and daunorubicin are anthracycline antibiotics that are natural products of certain soil fungi. Drug-induced free radicals may overwhelm the heart’s antioxidant defenses leading to cardiotoxicity. Daunorubicin and doxorubicin are administered IV, with care taken to prevent extravasation because local vesicant action may result. Ultimately, approximately 40% of daunorubicin and doxorubicin are metabolized (may result in hepatic dysfunction).
1. Clinical Uses. Daunorubicin is used primarily in the treatment of acute lymphocytic and myelocytic leukemia. Doxorubicin is one of the most active single drugs for treating metastatic adenocarcinoma of the breast, carcinoma of the bladder, bronchogenic carcinoma, metastatic thyroid carcinoma, oat cell carcinoma, and osteogenic carcinoma.
2. Side Effects. Cardiomyopathy and myelosuppression are side effects of the chemotherapeutic antibiotics.
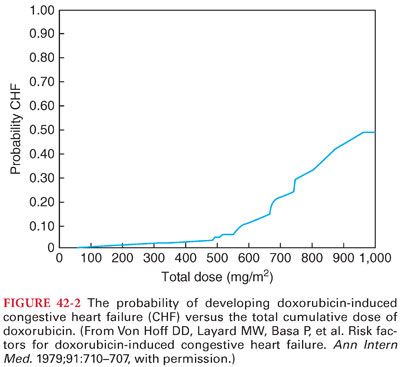
a. Cardiomyopathy is a unique dose-related and often irreversible side effect of the anthracycline antibiotics (congestive heart failure develops in <3% of patients with a cumulative dose of doxorubicin of <400 mg/m2, rising to 18% at 700 mg/m2) (Fig. 42-2). Previous treatment with anthracycline antibiotics may enhance myocardial depressant effects of anesthetic drugs even in patients with normal resting cardiac function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


