Cervical Squamous Cell Carcinoma
Epidemiology and Staging
Epidemiology
Cancer of the cervix is the second most common cancer in women worldwide after cancer of the breast. Each year, approximately 529,828 new cases are diagnosed worldwide.1 In the United States in 2012, cervical cancer was a distant third most common neoplasm of the female genital tract (12,170 cases, or about 14% of all genital cancers), after endometrium (47,130 cases) and ovary (22,280 cases).2 Breast cancer, by comparison, is 22 times more common (229,060 cases in the United States in 2012). Cervical cancer caused about 4220 deaths in 2012 in the United States (2.0 deaths/100,000 women). It is responsible for 1.6% of all deaths from neoplasia and 14% of all deaths from genital tract cancer. This compares with 39,510 deaths annually from breast cancer, 15,500 from ovarian cancer, and 8010 from cancer of the uterine corpus.2
The lifetime risk of a woman developing cervical cancer is 3% worldwide and 1.1% in the United States. The incidence rates for cervical cancer show a wide geographic variation, which is partially explained by differences in healthcare systems, intensity of screening programs, and exposure to major risk factors. A nearly eightfold difference exists between the lowest age-adjusted incidence rate (4.5/100,000 in western Asia) and the highest rate (34.5/100,000 women in eastern Africa).1 The age-adjusted incidence rate in the United States is 10.3/100,000 for African/American women, 11.4/100,000 for Hispanic women, but only 6.7/100,000 for non-Hispanic White women.3 During the past 50 years, both incidence and mortality rates for cervical cancer have declined precipitously in most developed countries. In the United States, the incidence has declined nearly 75% (from 34/100,000 in 1947) while the mortality rate has declined by more than 60%. The single most important factor in this decline is the success of screening with cervical cytology. Studies from both the Nordic countries and British Columbia demonstrate a significant reduction in cervical cancer incidence after only two cervical cytology tests.4 Repeated studies have shown the single most common factor associated with the development of cervical cancer is the history of not having undergone recent cytologic screening.5,6 The marked variations in cervical cancer rates in different regions of the globe relate to environmental, socioeconomic, and cultural factors, but, most importantly, to access to screening programs.
Staging
The most widely adopted staging system for cervical cancers worldwide is that of the International Federation of Gynecologists and Obstetricians (FIGO) (Table 11.1), which was last updated in 2009.7 This is a four-stage system that is in large part clinically determined, as opposed to pathologically determined. Stage I tumors are tumors that are confined to the uterus and are subdivided into two subcategories: those that are not macroscopically visible and invade 5 mm or less into the stroma and tumors that are macroscopically visible and/or invade more than 5 mm into the stroma. Stage II tumors invade beyond the uterus, but not to the pelvic sidewall or the lower third of the vagina. Stage III tumors extend to the pelvic sidewall and/or involve the lower third of the vagina and/or cause hydronephrosis or a nonfunctioning kidney. Stage IV tumors extend beyond the true pelvis or involve the mucosa of the bladder or rectum.
Table 11.1 2009 Modification of FIGO Staging of Carcinoma of the Cervix7
| Stage I | Cervical carcinoma confined to uterus (extension to the corpus should be disregarded) |
| IA | Invasive carcinoma that can be diagnosed only by microscopy with deepest invasion ≤5 mm and largest diameter ≤7.0 mm |
| IA1 | Measured stromal invasion ≤3.0 mm in depth and diameter of ≤7.0 mm |
| IA2 | Measured stromal invasion >3.0 mm but not greater than 5 mm with a diameter of ≤7.0 mm |
| IB | Clinically visible lesion confined to the cervix uteri or preclinical cancers greater than stage IA.* |
| IB1 | Clinically visible lesion ≤4 cm in greatest dimension |
| IB2 | Clinically visible lesion >4 cm in greatest dimension |
| Stage II | Cervical carcinoma invades beyond the uterus but not to the pelvic wall or to lower third of the vagina |
| IIA | Without parametrial invasion |
| IIA1 | Clinically visible lesion ≤4 cm in greatest dimension |
| IIA2 | Clinically visible lesion >4 cm in greatest dimension |
| IIB | With obvious parametrial invasion |
| Stage III | Tumor extends to the pelvic wall and/or involves lower third of the vagina and/or causes hydronephrosis or nonfunctioning kidney |
| IIIA | Tumor involves lower third of the vagina; no extension to pelvic wall |
| IIIB | Extension to pelvic wall and/or causes hydronephrosis or nonfunctioning kidney |
| Stage IV | The carcinoma has extended beyond the true pelvis or has involved (biopsy proven) the mucosa of the bladder or rectum. A bullous edema, as such, does not permit a case to be allotted to stage IV |
| IVA | Spread of the growth to adjacent organs |
| IVB | Spread to distant organs |
*All macroscopically visible lesions, even with superficial invasion, are allocated to stage IB. Invasion is limited to a measured stromal invasion with a maximal depth of 5 mm and horizontal extension of not >7 mm. Depth of invasion should not be >5 mm taken from the base of the epithelium of the original tissue, superficial or glandular. The depth of invasion should always be reported in millimeters, even in those cases with ‘early (minimal) stromal invasion’ (<1 mm). The involvement of vascular/lymphatic spaces should not change the stage allotment.
Types of Cervical Malignancies
The World Health Organization (WHO) currently recognizes three general histologic subtypes of cervical cancer: squamous cell carcinoma, adenocarcinoma, and ‘other epithelial carcinomas.’8 While squamous cell carcinoma accounted for upward of 90% of primary neoplasms several decades ago, the overall frequency has now dropped to about 60–70%.9,10 The various histologic variants of adenocarcinoma constitute much of the remainder of cervical cancers. The remaining primary malignancies of the cervix include sarcomas, lymphomas, and melanomas. Endometrial tumors frequently spread to the cervix, but it is unusual to find other tumors metastasizing to the cervix.
Superficially Invasive Squamous Cell Carcinoma
Superficially invasive squamous cell carcinoma (SISCCA), or ‘microinvasive carcinoma,’ a concept introduced over 50 years ago, refers to cancer of the cervix that demonstrates a minimal degree of stromal invasion, and as such has a prognosis much better than that of more invasive cervical carcinomas. In 2012, lower anogenital squamous terminology (LAST) for human papillomavirus (HPV)-associated lesions (LAST consensus recommendations by the College of American Pathologists and American Society of Clinical Pathologists; CAP/ASCP) unified microinvasive lesions under the moniker ‘superficially invasive squamous cell carcinoma,’ further designated by site-specific definitions. Thus, SISCCA of the cervix is defined as invasion ≤3 mm from the basement membrane point of origin, and horizontal spread ≤7mm.92 The critical point with regards to SISCCA is that these tumors, although locally invasive, present a negligible risk for having extended beyond the cervical conization specimen being examined, or of having metastasized to regional lymph nodes. As a result, patients with cervical SISCCA can be treated in a less radical manner without jeopardizing their chance for curative resection than women with more deeply invasive carcinomas. This is important because SISCCA accounts for about 20% of all cervical cancers and the majority of cases occur in women aged 35–46 years.11,12 SISCCA is now most often diagnosed in women presenting with abnormal smears interpreted as high-grade squamous intraepithelial lesion (HSIL).
Depth of Invasion
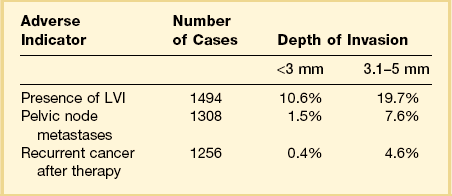
Among the many literature reports on SISCCA, whether presenting the findings of an individual series of patients or meta-analyses of all published articles, there is a general agreement that depth of invasion is a key prognostic indicator. Tumors that invade <1 mm into the underlying stroma are virtually never associated with lymph node metastases, although exemptions have been occasionally reported.13 Moreover, if we take 50–100 sections from a conization specimen performed for HSIL we are much more likely to find small epithelial buds extending <1 mm into the stroma than if we take only 10–15 sections. However, excellent clinical outcomes are achieved in both settings.7 Thus there is uniform agreement that tumors with very superficial stromal invasion (<1 mm) can be safely treated with conservative measures. There is more controversy with respect to the management of tumors with 1–5 mm of invasion. Although most studies have shown that the risk of lymph node metastases and the development of recurrent disease after conservative treatment are relatively low for carcinomas that invade ≤5 mm the risk is clearly not zero. For example, one study examining lesions invading <3 mm found that the recurrence rate was 6% during the 10 year follow-up period.14 Clinicopathologic analyses of carcinomas that invade ≤5 mm have shown a distinct difference between tumors invading ≤3 mm and those with 3–5 mm of invasion (Table 11.2).15–25 Tumors that invade to a depth of 3–5 mm are associated with lymph node metastases in about 8% of cases whereas those that invade ≤3 mm have only a 1–2% risk of lymph node metastases. The threshold of 3 mm invasion has now been formally adopted by the LAST group to define the maximum depth of invasion in SISCCA of the cervix.
Table 11.2 Impact of Depth of Invasion on Nodal Status, LVI, and Recurrence15–25 Modified from sources.
Tumor Volume and Lateral Extent of Spread
Although, as discussed above, it is generally accepted that depth of the invasion correlates with prognosis, other studies have documented that the tumor volume might be an even better prognostic measure. In order to accurately determine tumor volume, an excisional specimen needs to be serially step sectioned. When this is done and the distance between the sections is known, tumor volume can be calculated. This approach was first advocated by Burghardt and Holtzer,26 who reported no pelvic lymph node metastases in patients with <420 mm3 of cancer unless lymphovascular space involvement was identified. However, performing serial step sections to assess tumor volume is not practical for most laboratories. Thus FIGO has recommended that the greatest lateral extent of the tumor be measured as a surrogate for tumor volume. The usefulness of lateral tumor extension as a prognosticator was shown in an analysis of 402 women with squamous cell carcinomas by Takeshima et al.27 In this study, the incidence of nodal metastases was only 1.2% among patients with ≤3 mm of invasion but increased to almost 7% among women with 3–5 mm of invasion. However, in both groups, almost all recurrences occurred in women with >7 mm of horizontal spread. Based on these and other data showing the importance of lateral tumor extension, the FIGO staging system now requires stage IA tumors to be ≤7 mm in greatest diameter. This was reaffirmed by the LAST group, which defines SISCCA of the cervix as having no more than 7 mm in maximal extent.28
Lymphovascular Invasion
The role of lymphovascular invasion (LVI) as a prognostic indicator is more controversial than that of the depth of invasion and lateral extent of tumor. Although it would seem reasonable to assume that a tumor with LVI is more likely to have lymph node metastases than one without, the data are conflicting. In part, this stems from the relatively poor reproducibility of a pathologic diagnosis of LVI, especially when not extensive. It is well documented that the stroma in which foci of invasion lie can retract during preparation of the tissue sections for microscopic examination. A clear space can easily be mistaken for LVI. Another problem with the reporting of LVI is the fact that the finding is contingent on the number of sections that are examined. In one study serial step sections were examined from 30 cervices diagnosed with cervical carcinoma having only 2–5 mm of invasion. Thirty percent of the women had ‘capillary-like space’ involvement based on the first cut of tissue from the blocks, but this increased to 57% when the serial step sections were examined. All of the women in this study had been treated with radical hysterectomy with lymph node dissection. No lymph node metastases were found, regardless of the presence or absence of LVI. This study concluded that the presence of tumor in lymphatic spaces was of no value by itself in predicting which patients are likely to have lymph node metastases.29 However, other studies have reported that the presence of LVI is an important prognostic indicator.30,31 For example, a recent literature review of carcinomas with <3 mm of invasion reported recurrent cancer in 3.1% (3 of 96) of conservatively managed patients with LVI versus only 0.6% (3 of 486) of those without LVI.32 Overall, LVI has been reported in 0–10% of carcinomas invading <1 mm and 3–30% of those invading 1–3 mm.
Morphologic Features
Stromal Invasion
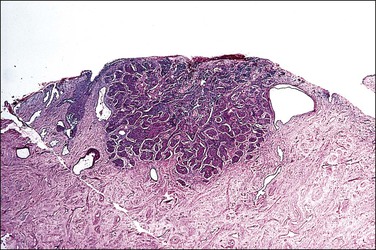
A diagnosis of SISCCA is made based on finding buds or tongues of malignant cells penetrating the basement membrane and extending into the stroma. The earliest stage at which invasion can be recognized is when a well-defined, tiny bud of invasive cells emanates from the base of HSIL on the surface of the cervix or in endocervical glands (Figure 11.1). The bud projects into the stroma, clearly having disrupted the adjacent smooth basement membrane. In one large series a single bud accounted for one-third of all the microinvasive cancers.33 However, in other cases, multiple small buds and sometimes individual tumor cells extend into the stroma.
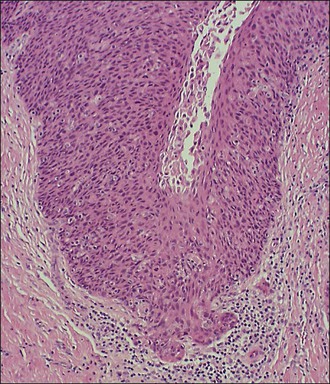
Figure 11.1 Microinvasive carcinoma. A tiny focus of stromal invasion that is barely perceptible arises from an extensive HSIL that fills an endocervical gland.
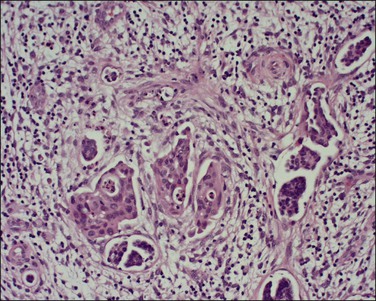
A number of different histologic patterns of SISCCA have been described. In very early foci of invasion the tumor cells are often separated by <1 mm from the nearest involved surface or glandular basement membrane. Cases with <1 mm tumor invasion were previously referred to as ‘early stromal invasion,’ which was the original definition of the FIGO substage IA1 in the 1985 FIGO classification. Another common pattern of growth is when microinvasive cancers appear as tiny nests of cells extending into the stroma that are totally separated from the overlying surface (Figures 11.2 and 11.3). This pattern of invasion has been referred to as a ‘spray bud’ pattern. The spray bud pattern of growth is usually <1–2 mm deep. As the foci of invasion become larger, the growths become broader and longer and eventually, as the tumor becomes more advanced, a ‘confluent’ pattern develops (Figure 11.4). In these larger lesions the invasive foci often form intertwining cords, much like thick tangled roots of a tree. It is important to stress that reproducible definitions of different patterns of invasion such as spray bud and confluent have proven difficult to develop and, not surprisingly, most studies have shown that the pattern of invasion does not influence clinical outcome independently of depth of invasion.30
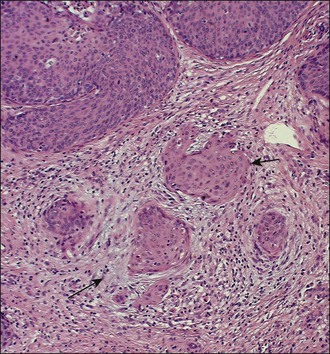
Figure 11.2 Microinvasive carcinoma. A focus of stromal invasion (arrows) that is separated by <1 mm from the overlying HSIL. In this instance multiple small tumor nests are totally separated from the surface epithelium.
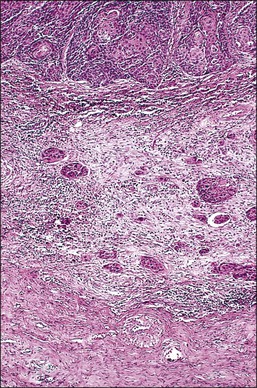
Figure 11.3 Microinvasive carcinoma, spray bud pattern. At high magnification, numerous clusters composed of small numbers of tumor cells are present in a desmoplastic stroma with a mild inflammatory infiltrate. Stromal retraction secondary to processing artifact creates the false impression of lymphovascular space invasion.
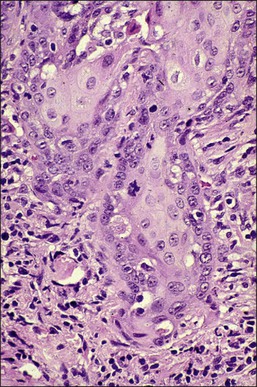
It is frequently difficult to determine whether a given focus represents SISCCA or instead is HSIL that extends into endocervical glands. There are four qualitative histopathologic features that can help with the diagnosis of SISCCA, in addition to measured limits of extent (Table 11.3).28 The first is presence of an epithelial margin that can be described as irregular, ragged, or even scalloped in appearance (Figure 11.5). This irregular margin develops because the adjacent smooth basement membrane has been disrupted. The second histopathologic feature is increased cellular maturation compared to the adjacent HSIL. The cells in the invasive foci often have abundant eosinophilic cytoplasm and are paradoxically keratinized (Figure 11.6). The third feature is that the nuclei of the invasive cells frequently show clearing of the chromatin and develop prominent nucleoli (Figure 11.7). The fourth feature is a stromal reaction to the SISCCA. Invasive foci are often surrounded by a prominent lymphoplasmacytic infiltrate and the stroma frequently has a desmoplastic response (Figure 11.3).
Table 11.3 Histopathologic Features of SISCCA
Qualitative Features:
Irregular, ragged, or even scalloped epithelial margin
Increased cellular maturation compared with adjacent HSIL
Nuclei of invasive cells show clearing of chromatin and prominent nucleoli
Stromal reaction to tumor with desmoplasia and prominent lymphocytic infiltrate
Quantitative Features28
Depth of invasion ≤3 mm from basement membrane of point of origin
Horizontal spread ≤7 mm maximal extent
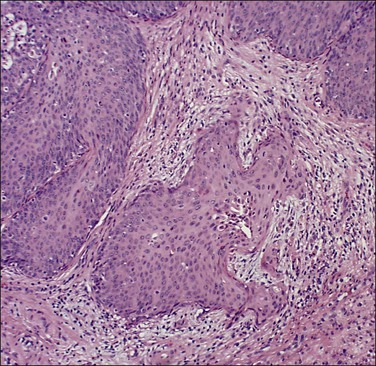
Figure 11.5 Microinvasive carcinoma. The invasive foci have an irregular or ‘scalloped’ margin that clearly distinguishes it from the sharp margin of the adjacent HSIL.
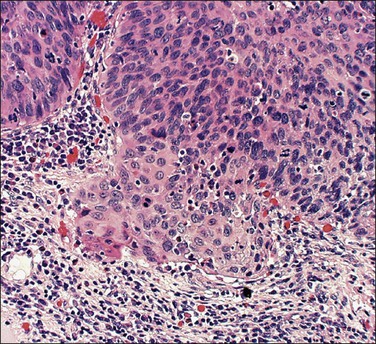
Figure 11.6 Microinvasive carcinoma. Cells in the invasive foci have developed abundant eosinophilic cytoplasm compared to the adjacent HSIL. Individual dyskeratotic cells are present in the invasive foci.
Measuring Depth of Invasion and Lateral Extent of Spread
Of all the variables evaluated during the assessment of SISCCA, depth of invasion is the one that is universally considered the easiest to measure and the most objective. The interobserver variability in assessing depth of invasion is also considered to be the lowest among the various features considered to have prognostic value. In order to accurately determine the maximum depth of invasion, loop electrosurgical excision procedure (LEEP) and conization specimens should be sectioned along the long axis of the cervical canal in a clockwise fashion while trying to avoid tangential sections, which invariably result in inaccurate measurement of the depth of invasion. The current FIGO/LAST staging method recommends that measurement of depth of invasion be made from the base of the epithelium where invasion occurs to the deepest point of carcinoma in a vertical line. In most SISCCAs, invasion either occurs from the base of the surface squamous epithelium or originates from both the surface epithelium and endocervical glands simultaneously. In such cases, measurement should be made from the base of the surface epithelium involved by HSIL. In some cases invasion is limited to the periphery of a few endocervical glands without surface involvement. In these cases, measurement is made from the base of the glands to the deepest point. The deepest measurement should be reported when multiple foci of SISCCA are present.
Assessing Presence of LVI
Even though the 2009 FIGO staging system does not take into account the presence or absence of LVI, most gynecologic oncologists want to know this information so that treatment can be individualized. Therefore the pathologist should always provide this information in the pathology report. Lymphatic space involvement was defined back in the 1960s as endothelial-lined (capillary-like) spaces containing tumor cells that are contiguous with the stroma. Until the recent introduction of the antibody D2-40 that specifically identifies lymphatic vessels, it was impractical to tell the difference between lymphatic and blood vessels under microscopy. Therefore the term lymphovascular space involvement, or invasion, came into widespread use when discussing cervical small vessel invasion by tumor, be it capillary or lymphatic. Identification of LVI, especially when only several foci are present, can be quite challenging. There is general agreement that LVI should be diagnosed whenever tumor is seen within endothelial-lined spaces at the leading edge of the tumor or beyond (Figure 11.8). Although it is preferable to identify tumor cells attached to the endothelium, this is not always possible. It is well documented that the stroma in which foci of invasion lie can retract during preparation of the tissue sections for microscopic examination. A clear space can easily be mistaken for LVI. Various immunohistochemical markers such as CD34, a marker for endothelium, and D2-40, a marker of lymphatic epithelium, can assist in identifying LVI in some instances. However, it should be remembered that there are a variety of markers available and the results obtained with them can be discordant. Moreover, it is unclear whether LVI, as determined by immunohistochemical markers, correlates with clinical outcomes.34 Therefore, the standard for assessing the presence or absence of LVI in the cervix remains H&E staining alone.
Assessing Surgical Margins
Although the 2009 FIGO staging system does not take into account the status of the surgical margins of a conization or LEEP specimen, assessment of the surgical margins of excision is one of the most important contributions of the pathologist to management of women with SISCCA. This is because women with either HSIL or SISCCA on the surgical margin are much more likely to have a residual invasive cervical cancer than are women with clear margins. Inking of LEEP specimens is optional since a thermal artifact is universally present in the resection margin. However, a cold-knife cone requires application of ink at the margins, preferably one color at the endocervical and another on peripheral or deep margins.35
Diagnosis
SISCCA is a diagnosis based almost wholly on microscopic examination and it usually is an incidental finding in either a cold-knife conization or LEEP specimen obtained for the treatment of HSIL. Most experts believe that a diagnosis of SISCCA should only be made on a LEEP or conization specimen for which the margins of excision are free of both HSIL and SISCCA. This is because the depth of invasion seen on a cervical biopsy specimen or a LEEP/conization specimen with a positive margin may not represent the maximal depth of invasion. SISCCA is a condition that is easily misdiagnosed. Almost 50% of cases diagnosed as SISCCA during routine clinical care are either cases of carcinoma in situ that have been overcalled or cases of invasive carcinoma that have been missed. For example, in a series of 265 cases of SISCCA submitted to a reference pathology panel as part of a Gynecologic Oncology Group (GOG) study, 132 (approximately 50%) were reclassified as something other than SISCCA.15 Similarly, in a UK study of 286 cases of SISCCA that underwent pathologic review, 41% were incorrectly diagnosed.36
The utility of immunohistochemistry in the diagnosis of SISCCA has been evaluated for a double-staining method combining collagen IV or laminin antibodies to identify the basement membrane and pan cytokeratin antibodies to identify invasive squamous cells.37 Although in some equivocal cases this approach appears to be useful, since it identified clear-cut invasion in 4 of 10 cases originally reported as ‘suspicious for invasion,’ it does not always clarify whether or not invasion is actually present.
Cytologic Findings
The cytology of microinvasive carcinoma is controversial. Most cytologists believe that the distinction between HSIL and the earliest stages of invasion cannot be made on cell appearances alone, and for this reason SISCCA is not included as a specific entity in the Bethesda System.38
Clinical Management
The treatment for stage IA1 lesions is typically a LEEP, cold-knife conization, or a simple hysterectomy. With these approaches the cure rate approaches 100%.39,40 The presence or absence of LVI as well as the exact depth of invasion will impact the treatment approach for stage IA1 tumors. Tumors with <1 mm of invasion almost never have pelvic lymph node metastasis whereas tumors with 1–3 mm invasion or with LVI have a low, but measurable, rate of nodal involvement. If fertility is not a consideration and a woman has adverse prognostic features such as LVI or involved margins, simple hysterectomy is the standard form of definitive treatment. The management of stage IA2 tumors is more controversial. In the United States, women with stage IA2 tumors and LVI are usually not considered candidates for conservative management and they are typically treated with a modified radical hysterectomy and pelvic lymphadenectomy. There is a growing trend, however, to tailor the management for patients desirous of maintaining fertility who present with stage IA2 tumors without LVI and with negative conization margins.41
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree