Cervical Benign and Non-Neoplastic Conditions
Physiologic Changes in the Cervix and the Formation of the Transformation Zone
Inflammatory Cervicitis and Reactive Changes
Lesions of the Endocervical Glandular Epithelium
Lesions Related to Exogenous Stimuli
Other Non-Neoplastic Conditions
Normal Structure
Anatomy
The cervical canal connects the uterine cavity to the vagina and it is flattened from front to back. The external os is a rather imprecise landmark, indicating the point at which the cervical canal opens into the vagina. Tissue that lies outside the external os, on the vaginal portion of the cervix, is referred to as ectocervix or exocervix. That located above the external os, within the canal, is endocervix (Figures 8.1 and 8.2). While strictly incorrect, many histopathologists use these terms to describe the type of tissue found rather than its position. Thus, a biopsy consisting of stroma, glandular surface epithelium, and crypts may be described as endocervical, even though the material may come from a position on the vaginal portion of the cervix, well outside the external os.
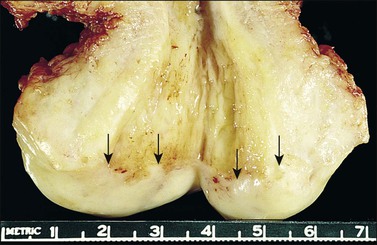
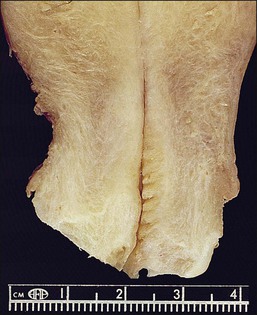
Figure 8.1 Normal cervix. The specimen has been opened to show the rough appearance of the cervical canal in contrast to the smoothness of the ectocervix. The squamocolumnar junction (arrows) is clearly visible.
Native (Original) Squamous Epithelium
The epithelium covering the cervix is initially of two types, which are laid down during embryologic development. These can be described as the ‘native’ (or original) squamous and columnar epithelia of the cervix. The interface between these two epithelium types is dynamic, and later is the site of the transformation zone.
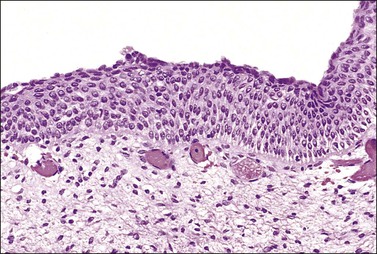
The squamous epithelium is stratified and nonkeratinizing. It is under the influence of ovarian hormones just as is the vaginal epithelium, although the changes in response to hormonal stimuli are less marked than those encountered in the vagina. The normal appearance in a woman of reproductive age is shown in Figure 8.3.
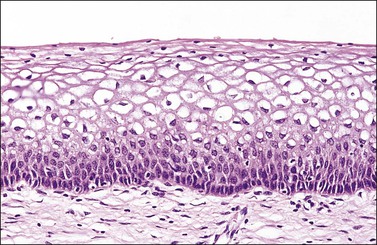
Figure 8.3 Original squamous epithelium in a woman of reproductive age. Maturation of the squamous epithelium is obvious. The basal layer, parabasal zone, intermediate zone, and superficial layer can be clearly distinguished.
Cytologic Correlation
Parabasal Squamous Cells.
Parabasal cells exhibit a round shape and dense, green/blue cytoplasm. The large nucleus, which approximates 80–90% of the total cell size, is darkly stained with evenly distributed chromatin. Since these cells are immature squamous cells, they are generally seen in smears from postmenopausal women (Figure 8.4) or postpartum.
Intermediate Squamous Cells.
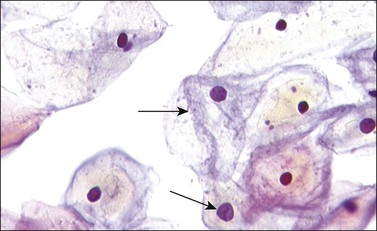
As the squamous epithelium responds to hormonal stimuli, the individual cells mature and the intermediate squamous cells reflect this change, both with an increase in the amount of cytoplasm in relation to the nucleus and in terms of total cell size. The cytoplasm becomes more transparent and tends to stain pale blue. Occasionally an area around the nucleus stains yellow, denoting the presence of glycogen. The nucleus is round with pale-staining, finely granular chromatin (Figure 8.5).
Superficial Squamous Cells.
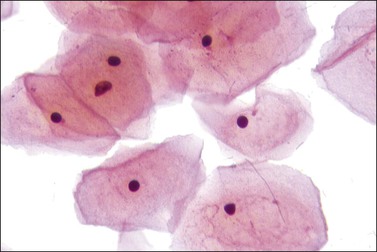
With progressing maturation the superficial squamous cells predominate. The cells are large and polyhedral with angled edges. The pink-staining cytoplasm is virtually transparent, and the pyknotic nuclei are small and densely stained with little or no apparent chromatin structure (Figure 8.6). Keratinization does not occur normally in the cervix, although it is associated with several pathologic conditions.
Hormonal Influences on Squamous Epithelium
After ovulation, when circulating progesterone counteracts the effect of estrogen on the epithelium, the cervical–vaginal smear shows a predominance of intermediate cells and a decreased number of superficial cells. However, this epithelium is often difficult to appreciate in histologic sections. After menopause, when there is a deficiency of ovarian hormones, the squamous epithelium becomes much thinner, and there is no maturation beyond the parabasal level. The thin epithelium is therefore composed entirely of parabasal cells and may appear quite uniform on section (Figure 8.7). The lack of a mature epithelium, coupled with the high nuclear to cytoplasmic ratio that the parabasal cells exhibit, can make the distinction from squamous intraepithelial lesion (SIL) difficult (see Chapter 10). However, the regular nuclear contour, the uniform chromatin pattern, and lack of conspicuous mitotic activity point to the correct diagnosis. In difficult cases, the use of immunohistochemical studies will facilitate the correct diagnosis. Atrophic squamous epithelium exhibits little or no proliferative activity, with only focal staining of the basal and parabasal cells with Ki-67 (MIB1). In addition, p16 staining is either negative or minimal.1 The appearances before puberty are similar (Figure 8.8).
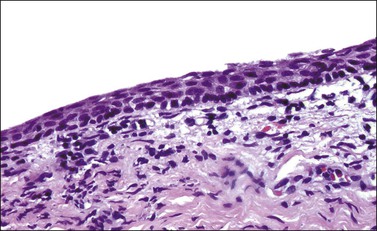
Figure 8.7 Atrophic squamous epithelium. In a postmenopausal woman, the epithelium is thin and the cells do not mature beyond the parabasal stage.
Cytologic Correlation
Postmenopausal Atrophy.
The postmenopausal smear pattern shows a predominance of parabasal and occasional intermediate cell types with no cyclic changes (Figure 8.4). Endocervical cells are frequently not identified. Although a similar pattern is seen postpartum, squamous metaplasia and regenerative changes and endocervical cells are more frequently noted.
Endometrial Cells.
Menstrual smears contain increased numbers of leukocytes and varying numbers of red blood cells and endometrial cells. The endometrial cells frequently are found in small darkly stained groups but can be seen in strings or as individual cells, particularly in the late menstrual phase (Figure 8.9). Endometrial cells approximate the size of a neutrophil, with darkly stained coarse chromatin. Nuclei, although normally round, with darkly stained coarse chromatin, often show some irregularity in shape, which may reflect degenerative change. The small amount of cytoplasm usually takes a basophilic stain. Endometrial cells should not normally be seen in a cervical smear after days 10–12 of the menstrual cycle. Not surprisingly, a menstrual smear will frequently be deemed inadequate for reliable assessment, since much cell detail can be obscured.
Basal Cell Hyperplasia
In this condition the basal layer and adjacent part of the parabasal layer form an unusually well-defined stratum that is increased in thickness and is conspicuous because of both nuclear enlargement and cytoplasmic basophilia (Figure 8.10). The oval basal and parabasal nuclei are usually oriented vertically and the picket-fence appearance of a single layer is lost. Nuclear pleomorphism and hyperchromasia are absent. The cells above this stratum are mature and stratified. This condition is usually without import.
Squamous Cell Hyperplasia
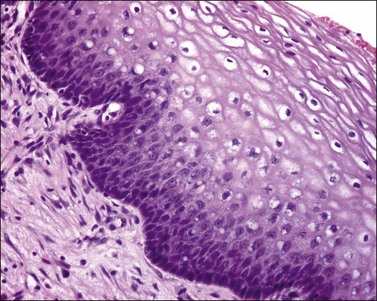
The squamous epithelium of the ectocervix becomes thickened when there is uterovaginal prolapse. In these circumstances the epithelium shows acanthosis and there may be an irregularity of the epithelial–stromal junction resembling the rete ridges of the skin. Frequently, there is also hyperkeratosis with/without parakeratosis (Figure 8.11), which may or may not have a granular layer. These changes are the response to mechanical stimulation and take place in the absence of hormonal stimulation. Most prolapses are seen in women after menopause.
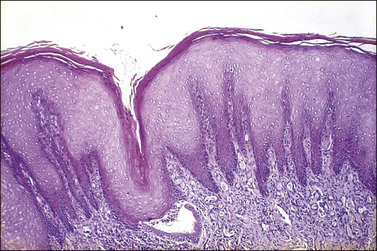
Figure 8.11 Squamous hyperplasia. The epithelium is acanthotic with hyperkeratosis and parakeratosis.
Native (Original) Cervical Glandular Epithelium
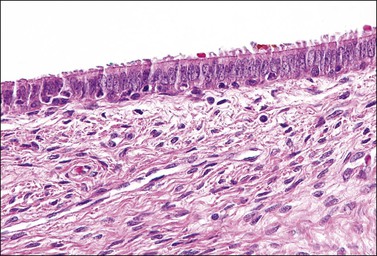
Columnar cells line the endocervical canal. The majority of these are tall, thin, mucus-secreting cells with basal nuclei, presenting the familiar picket-fence appearance (Figure 8.12). Ciliated cells are seen in the upper endocervix (Figure 8.13). The epithelium takes the form of villous processes (Figure 8.14) that are seen most dramatically at colposcopy. Although these grape-like villi are characteristic of endocervical tissue colposcopically, they are not always easily seen, particularly in older women. They are, at all ages, more striking when adjacent to the squamocolumnar junction. A further complexity of the endocervical epithelium is the formation of crypts, which are tunnels lined by mucin-secreting epithelium passing into the substance of the cervix, usually to a depth of no more than 3 mm, but on occasion to as much as 1 cm (Figure 8.15). These structures are commonly referred to as ‘glands,’ but as shown by serial sections they are a system of clefts that may become occluded and thereby form blind-ending tunnels. An awareness that these crypts are present is important when SIL affects the cervix (see Chapter 10). The columnar epithelium of the endocervix meets the squamous epithelium of the ectocervix at the squamocolumnar junction (Figure 8.16). The cytoplasm of the columnar cells stains with mucicarmine and Alcian blue, and is reactive for various mucin antigens, e.g., MUC1, MUC4, and MUC5AC.2
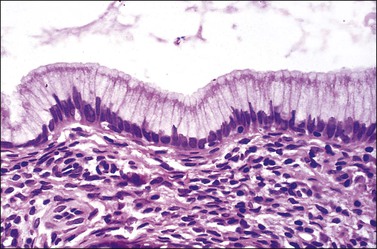
Figure 8.12 Normal endocervical epithelium. The cells are columnar, contain mucin, and the nuclei are basally located.
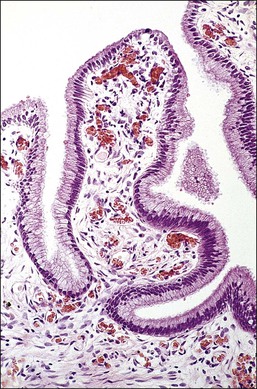
Figure 8.14 Endocervical villi. The papillae exhibit a central fibrous core with blood vessels and a mantle of endocervical mucinous columnar cells.
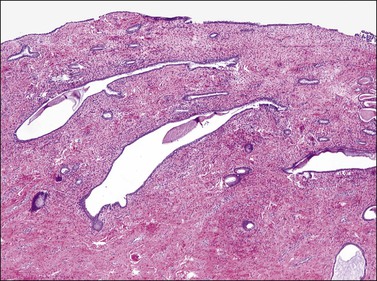
Figure 8.15 Endocervical crypts. Tunnels that are lined by mucinous endocervical columnar epithelium penetrate into the endocervical fibromuscular stroma.
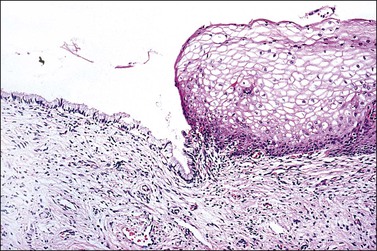
Figure 8.16 Squamocolumnar junction. There is an abrupt transition from squamous to columnar epithelium.
Cytologic Correlation
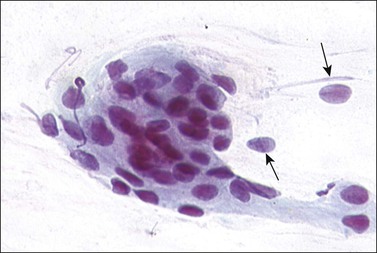
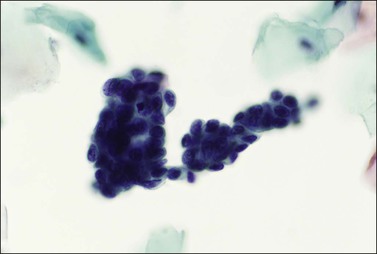
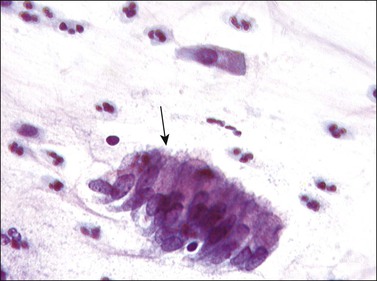
Frequently referred to as ‘endocervical cells,’ glandular cells of endocervical origin are easily identified in cervical smears. They usually occur in sheets or small groups, although occasionally whole or partial fragments of villi are seen. The nuclei show a finely granular chromatin, sometimes with small nucleoli, and the cells have a cylindrical shape. The pale blue/gray cytoplasm is often vacuolated with poorly defined cell borders (Figure 8.17). Cilia can occasionally be seen with well-defined terminal plates (Figure 8.18). When viewed end on, the round nuclei are centrally placed within the cytoplasm and the close proximity of the cells resembles a honeycomb appearance. Endocervical cells are approximately four to five times larger than endometrial cells, so misinterpretation is uncommon.
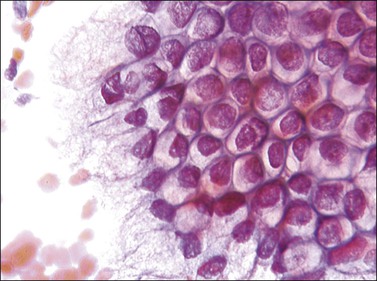
Figure 8.17 Endocervical cells in a cervical smear. Honeycomb arrangement of the cells and mucin in the cytoplasm.
Physiologic Changes in the Cervix and the Formation of the Transformation Zone
Fundamental to understanding cervical epithelial abnormalities is the formation of the transformation zone. It is in this area of the cervix that SIL and eventually invasive squamous cell carcinoma most commonly develop. The process is represented diagrammatically in Figures 8.19–8.22. Before puberty is reached, the squamocolumnar junction is situated at or near the external cervical os (Figure 8.19). During adolescence the increasing levels of circulating ovarian hormones cause an increase in the bulk both of the body of the uterus and of the cervix, the latter growing proportionally more during this period. A consequence of this increased cervical size is its eversion outward, rather more markedly anteriorly and posteriorly than at the sides. As a result of this change in shape, the endocervical epithelium, together with the underlying crypts and stroma, come to lie on the vaginal portion of the cervix (Figure 8.20). The ‘ectopic’ endocervical epithelium thus situated on the ectocervix is red and rough. It is red because the columnar epithelium is one cell layer thick and essentially transparent to the rich network of underlying blood vessels. It is rough because of the villous appearance of the endocervical tissue.
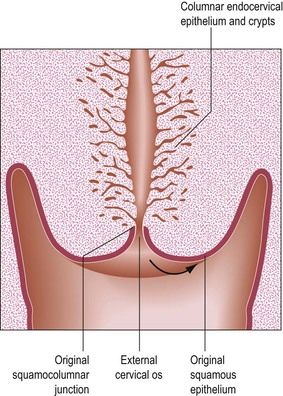
Figure 8.19 Prepubertal cervix. The squamocolumnar junction is situated at the external cervical os. The arrow shows the direction of the movement that takes place as a result of the increase in bulk of the cervix during adolescence.
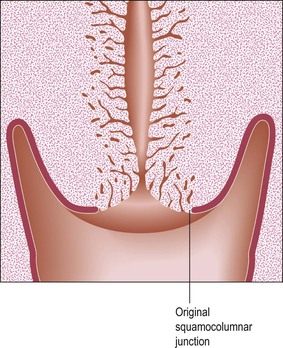
Figure 8.20 The process of eversion. On completion, endocervical columnar tissue lies on the vaginal surface of the cervix and is exposed to the vaginal environment.
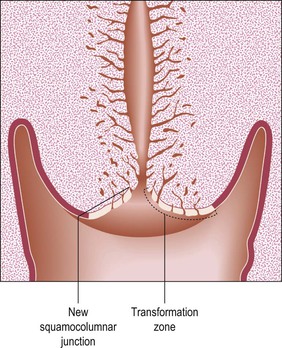
Figure 8.21 Postadolescent cervix. The acidity of the vaginal environment is one of the factors that encourage squamous metaplastic change, replacing the exposed columnar epithelium with squamous epithelium.
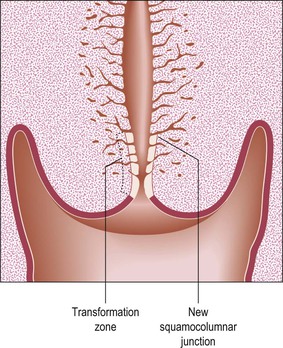
Figure 8.22 Postmenopausal cervix. At this time, cervical inversion occurs. This phenomenon is the reverse of eversion, which was so important in adolescence. The transformation zone is now drawn into the cervical canal, often making it inaccessible to colposcopic examination.
The process of eversion, which gives rise to the transformation zone (ectropion), occurs predominantly during adolescence. Eversion also occurs at other times, notably in the neonatal period and with each pregnancy, after which the squamocolumnar junction regresses to approximately the same position as before that pregnancy. The normal physiologic result of cervical eversion is replacement of the endocervical columnar epithelium by squamous epithelium through the process of squamous metaplasia (Figure 8.21). As menopause approaches, this process reverses, so that the transformation zone tends to pass back into the cervical canal. This process is called ‘inversion.’ In the postmenopausal woman, the whole transformation zone, whether typical or atypical, may be situated inside the canal and so out of sight both to the naked eye and to colposcopy (Figure 8.22).
Squamous Metaplasia
This mechanism of squamous metaplasia permits the much tougher and more resistant squamous epithelium to replace the highly specialized, fragile columnar cells of the endocervical epithelium normally present.
The columnar cells do not, of course, transform into squamous cells, as mature epithelial cells of one type cannot change to differentiate and become another cell type. The stimulus to squamous metaplasia in the cervix appears to be the increased acidity of the vaginal environment compared with that of the cervical canal. Figures 8.23–8.33 show stages in the evolution of a metaplastic squamous epithelium. Reserve cells are located beneath the columnar epithelial cells and they give origin to metaplastic squamous cells (Figure 8.23).
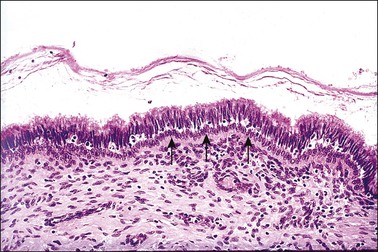
Figure 8.23 Reserve cells. A single row of reserve cells (arrows) is present beneath the columnar epithelium.
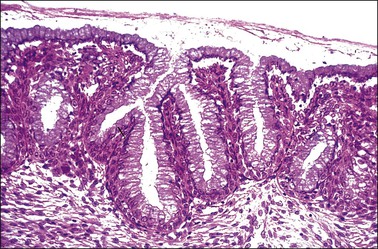
Figure 8.24 Reserve cell proliferation. The reserve cells proliferate to form several layers beneath the still intact columnar epithelium.
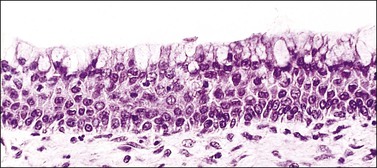
Figure 8.25 Immature squamous metaplasia. As the process progresses, the cells acquire more abundant eosinophilic cytoplasm, stratified into multiple layers, and appear as immature squamous cells lacking intracytoplasmic glycogen. Commonly, a layer of mucinous epithelium lies superficial to the metaplastic cells.
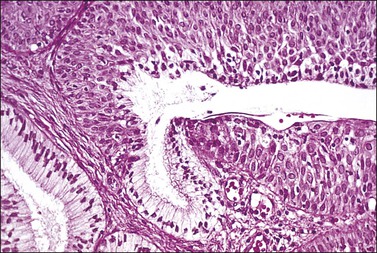
Figure 8.26 Immature squamous metaplasia. The lack of differentiation and maturation can cause confusion with intraepithelial neoplasia.
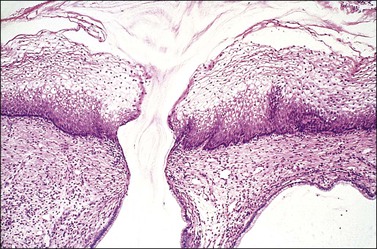
Figure 8.27 Mature squamous metaplasia. The mature metaplastic squamous epithelium here overlies gland crypts, lined by columnar epithelium. Mucin is being discharged from a gland opening.
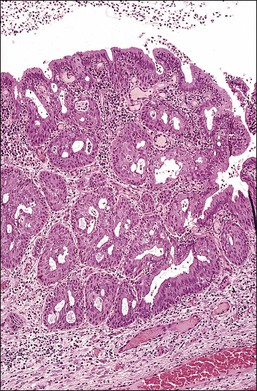
Figure 8.28 Squamous metaplasia in an early stage exhibiting hyperplasia of the reserve cells and immature cells. At this stage, the glandular tissue is only partially replaced. As the metaplastic process continues, the amount of residual glandular tissue will decrease and then disappear.
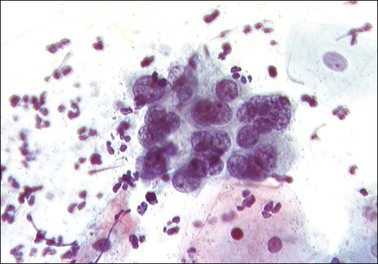
Figure 8.29 Early squamous metaplasia in a cervical smear. The cells, almost indistinguishable from endocervical cells, show clumped chromatin.
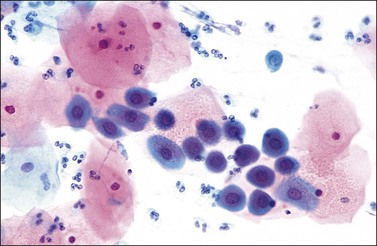
Figure 8.30 Squamous metaplasia in a cervical smear. Darkly stained, small nuclei within basophilic cytoplasm.
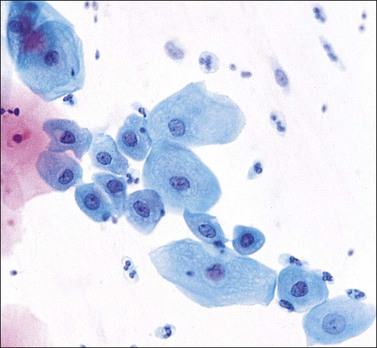
Figure 8.31 Squamous metaplasia in a cervical smear. Some cells show smaller nuclei with an increased amount of cytoplasm and a more squamoid appearance.
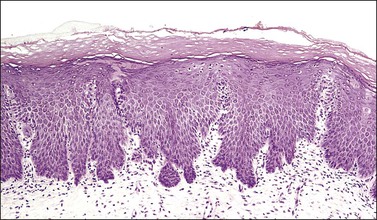
Figure 8.32 Congenital transformation zone. The epithelium has many features of metaplastic squamous epithelium. Highly characteristic is the irregular, dentate outline of the epithelial–stromal junction.
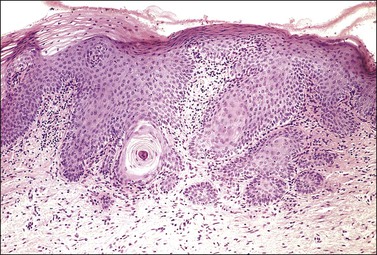
Figure 8.33 Congenital transformation zone. An obvious keratin pearl is present deep within the epithelium.
The origin of these reserve cells is from a population of cytokeratin 7-positive stem cells located at the transformation zone. It is this population of stem cells that replenishes basal reserve cells, generates the squamous metaplastic cells in the transformation zone, and is the stem cell of cervical SIL and carcinoma.3 Reserve cells first proliferate as a row of nondescript, small, round cells beneath the columnar epithelium, usually following the contour of the basement membrane (Figure 8.24). Incomplete and immature squamous metaplasia may show columnar cells not totally replaced by squamous cells (Figure 8.25).
As the process continues, the squamous cells make up the whole thickness of the epithelium and start their differentiation to maturity, eventually rendering the metaplastic epithelium indistinguishable from normal, mature, glycogenated squamous epithelium. Metaplastic squamous epithelium may be mistaken for SIL. Figure 8.26 illustrates this dilemma. The epithelium is composed of squamous cells that are immature up to the surface of the epithelium, a feature that is often considered an important diagnostic criterion in the diagnosis of SIL regardless of grade. However, the metaplastic nuclei are regular and round or oval, each with a single, prominent nucleolus, in contrast to SIL where pleomorphism is the rule. The nuclei in metaplastic epithelium lack hyperchromasia and mitotic figures are few, confined to the basal layers, and not abnormal. In addition, normal immature squamous cells are unreactive for p16, which is a surrogate marker for integrated high-risk human papillomavirus (HPV) DNA and almost always seen in high-grade SIL (HSIL).1,4–6
In its final state, mature metaplastic squamous epithelium may be histologically indistinguishable from the original squamous epithelium. The only clue to its origin within the transformation zone is the presence of underlying crypts with their mucinous epithelium (Figure 8.27). Squamous metaplasia occurs most notably at the time of adolescence and during pregnancy but may be found in the cervix of women who entered menopause long before.
Although squamous metaplasia is a change that mainly involves the surface epithelium of the cervix, the crypts are also commonly affected (Figure 8.28). Sometimes the crypt involvement is sufficiently marked to impart an initial impression of invasive carcinoma. In the cervix, the pathologist should be wary of entertaining a diagnosis of frank malignancy when there is a layer of mucinous columnar epithelium that overlies the questionably cancerous tissue.
Cytologic Correlation
An important aspect associated with recognizing squamous metaplasia cytologically is that metaplastic cells exhibit a wide variety of appearances. Within a single cervical smear, both immature and mature cell types are frequently seen together with the whole spectrum of change (Figures 8.29–8.31). From a cytologic perspective, the terms ‘immature’ and ‘mature’ have a slightly different meaning from that used by histopathologists. Cells arising from a histologically mature metaplastic epithelium will be virtually indistinguishable from mature squamous cells. The term mature as used by the cytologist describes cells that show some features of differentiation but still show some characteristics seen in immature metaplastic cells.
In the earliest phase of change, small round cells with a narrow rim of green-staining cytoplasm appear that closely resemble endocervical cells. The nuclei are often hyperchromatic with coarsely clumped chromatin. They are usually seen in small tight groups, a feature that makes recognition easier (Figure 8.29). The cells arising from an area of immature metaplasia are normally round in shape with large, darkly staining nuclei with a coarse chromatin pattern that may contain nucleoli or chromocenters. The cytoplasm is dense with a well-defined cell border and usually stains green (Figure 8.30). Differentiation from HSIL should in most instances be straightforward by attention to the absence of pleomorphism. However, immature metaplasia remains a source of error in diagnosis.
Mature Metaplasia.
As the metaplastic epithelium matures, the individual cells assume a squamoid appearance. The cytoplasm becomes more abundant and the cell appears oval or elongated. The cytoplasm may stain either blue or pink but it does not achieve the transparency of a mature squamous cell. Not infrequently the cytoplasm may be pulled out into tails and projections, the remnants of intercellular bridges. The nucleus becomes less dominant with a finer chromatin structure, but it always appears larger in comparison to the nucleus of a squamous cell (Figure 8.31).
The Congenital Transformation Zone (CTZ)
The CTZ is a variant of squamous metaplasia that exhibits incomplete maturation (Figure 8.32). The deeper layers show the same features as a maturing squamous metaplasia. The nuclei are large but regular, with prominent nucleoli. There is minimal pleomorphism, and mitotic figures, which are infrequent, can usually be found with diligent searching. A layer of hyperkeratosis or parakeratosis is often present, and this layer, when thick, corresponds to the leukoplakia that is a characteristic colposcopic feature of the CTZ. One of the most striking histologic features is the pattern of its lower margin, the epithelial–stromal junction. This is always irregularly dentate, presenting an appearance akin to the rete ridges of the skin. Sometimes the tips of these epithelial incursions into the stroma appear detached from the overlying epithelium, so that they may give the impression of invasive buds. This impression may be heightened when the centers of the processes undergo differentiation, so that a whorl of keratin is seen in the center (Figure 8.33). Attention to the cytologic features will show that the CTZ is not an invasive tumor. It is unusual for there to be any stromal reaction to the CTZ. Another cardinal feature is the absence of cytoplasmic glycogen (i.e., no ‘basket-weave’ pattern). This causes the epithelium to be iodine negative on examination, further attracting the attention of the colposcopist.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree