Cerebrospinal and Miscellaneous Fluids
CEREBROSPINAL FLUID
CEREBROSPINAL FLUID
Anatomic, Physiologic, and Clinical Considerations
The cerebrospinal fluid (CSF) is formed from circulating blood that is filtered though a complex epithelial organ, the choroid plexus, located within the ventricular system of the brain. The normal choroid plexus is a highly selective filter that regulates the chemical make-up of the CSF and maintains it at a constant level, independently of variations in the blood serum. The choroid plexus is also endowed with genes that prevent the passage of most toxic substances into the CSF. No blood cells, except for an occasional monocyte or lymphocyte, cross the choroid plexus, which constitutes a highly effective blood-brain barrier, ensuring optimal working conditions for the brain. The CSF is formed by the choroid plexus at a constant rate and is drained by the leptomeninges, resulting in constant volume of fluid, at 10 to 60 ml in children, depending on age, and 90 to 150 ml in adults.
Normal CSF is a crystal clear liquid containing only very few mononucleated blood cells and lower levels of glucose and proteins than the blood serum. The CSF bathes the entire internal ventricular system of the brain, its external surfaces, the cerebellum, and the spinal cord. The extracerebral CSF is contained between two epithelial meningeal membranes, the pia (lining the brain) and the arachnoid (lining the dura). Occasionally, cells derived from the choroid plexus, the pia, and the arachnoid may desquamate into the CSF. Normally, there are very few of these cells and they are difficult to identify.
Any increases in the number of cells in the CSF or changes in the glucose and protein levels invariably indicate a pathologic process. Enumeration and cytologic examination of the cells in the spinal fluid serve to clarify the nature of the disease.
Cytologic examination of the CSF is an important part of a complete neurologic evaluation of cancer patients and patients with the acquired immunodeficiency syndrome (AIDS), particularly if there is clinical evidence or suspicion of central nervous system (CNS) involvement. Also, patients with space-occupying lesions of the CNS of unknown nature should have the benefit of this examination. Cytologic evaluation of CSF has become an essential step in the follow-up of patients with lymphoma and leukemia and some small-cell tumors, such as oat cell carcinoma of pulmonary origin, because the therapy of these tumors has been revolutionized by this procedure (see below). An increasingly important application of cytology of the CSF is the diagnosis of infectious processes, particularly in patients with AIDS.
Methods of Securing CSF for Laboratory Investigation
Most samples of CSF sent to the laboratory are obtained by spinal tap and this chapter is essentially based on this type of sample. Another source of CSF is the cisterna magna and the ventricles of the brain, which are sometimes more informative, particularly in reference to primary brain tumors. Securing such samples requires intervention by a skilled neurosurgeon. Some of the data in the literature are based on a mixture of samples obtained by various methods. Whenever possible, these differences are stressed in the text.
Laboratory Techniques
Because the samples of CSF are usually small, rarely more than 10 ml, impeccable laboratory techniques are essential if the cytologic evaluation of the fluid is to be successful. Regardless of the method used, cell loss must be avoided. Sedimentation techniques offer the best overview of the cell content. Hematologic stains and techniques of cell preparation are helpful in cell identification in samples rich in mononuclear cells, particularly in lymphomas and leukemias. The various technical approaches to the processing of CSF are discussed in Chapter 44. Classification of lymphocytes by flow cytometry may be successfully conducted on cell-rich specimens (see Chaps. 31 and 47). Immunocytochemistry is occasionally helpful in identifying the origin of metastases in patients with past history of two malignant tumors (see Chap. 45). Various methods of CSF investigation by molecular biologic techniques are described in text.
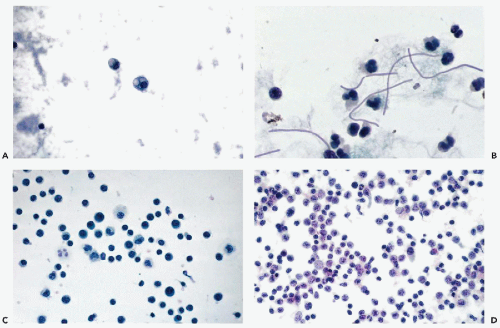
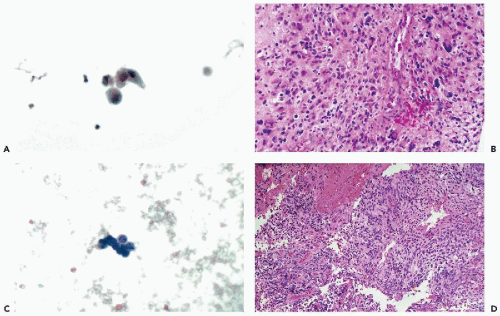
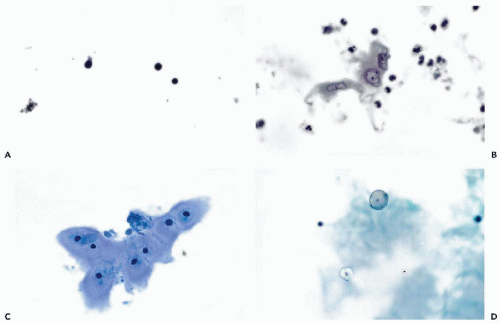 Figure 27-1 Benign findings in cerebrospinal fluid (CSF) in cytocentrifuge preparations. A. Normal CSF contains only a few lymphocytes and monocytes, the latter characterized by more abundant cytoplasm. B. CSF containing leukocytes and a large epithelial cell, probably of meningeal origin. C. Chondrocytes aspirated from nucleus pulposus. D. Powder crystal mimicking cryptococcus (compare with Fig. 27-3). |
Special Diagnostic Procedures
Kajikawa et al (1977) used CSF to establish primary shortterm tissue cultures of various primary tumors of the central nervous system. The procedure was successful in a number of instances and the identification of tumor type was easier in the culture than in the original CSF.
Cells and Acellular Components in the Absence of Disease
Cells
With the cell collection techniques used in this laboratory, notably cytocentrifugation and Papanicolaou stain (see Chap. 44), only a very few small lymphocytes and monocytes are observed in the CSF of adults. The monocytes have a somewhat larger, more open, sometimes indented nucleus and a slightly larger rim of cytoplasm. Thus, only two types of cells and two nuclear forms are normally observed (Fig. 27-1A). By using a careful cell collection technique,
Dyken (1975) found that the cell count is higher in neonates (less than 1 month in age) than in older persons. For the neonates, the average count was 10.17 ± 8.45 cells per cubic millimeter, and for older persons, 2.59 ± 1.73 cells per cubic millimeter. There were also some differences in the differential counts: monocytes were the most prevalent cell in neonates, whereas in older persons lymphocytes were most commonly observed. In a subsequent study, Dalens et al (1982) confirmed the original observations; in addition, cells of the choroid plexus and the arachnoid were noted as a transient phenomenon during the first week of life. The presence of these cells is probably caused by slight brain trauma sustained during birth.
Dyken (1975) found that the cell count is higher in neonates (less than 1 month in age) than in older persons. For the neonates, the average count was 10.17 ± 8.45 cells per cubic millimeter, and for older persons, 2.59 ± 1.73 cells per cubic millimeter. There were also some differences in the differential counts: monocytes were the most prevalent cell in neonates, whereas in older persons lymphocytes were most commonly observed. In a subsequent study, Dalens et al (1982) confirmed the original observations; in addition, cells of the choroid plexus and the arachnoid were noted as a transient phenomenon during the first week of life. The presence of these cells is probably caused by slight brain trauma sustained during birth.
In our experience, the identification of normal cells derived from the brain, such as choroid plexus and ependymal cells, is extremely difficult in adult patients in CSF obtained by spinal tap. Kline (1962) and Naylor (1961, 1964) described ependymal cells as small cuboidal cells arranged in rows or clusters; their occurrence may be expected, mainly in fluid aspirated directly from the cerebral ventricles or the cisterna magna, or in direct samples of the brain, described in Chapter 39. Wertlake et al (1972) also observed occasional pia-arachnoid cells, resembling mesothelial cells and, occasionally, cells interpreted as astrocytes in ventricular fluid. Elongated cells, described by Kline as originating from meningeal lining, have not been observed by us in normal spinal fluid. Kölmel (1976) observed such cells after pneumoencephalography. However, we did occasionally observe in cases of meningitis, fairly large epithelial cells with transparent cytoplasm and degenerated nuclei that we assumed were of meningeal origin (Fig. 27-1B). The cytologic-histologic correlation of these rare cells has been carried out by McGarry et al (1969).
Bone marrow cells may be observed when the tap needle inadvertently enters a vertebral body. Megakaryocytes are usually a conspicuous component of such faulty taps.
Chondrocytes (Fig. 27-1C) occur when the tap needle incidentally enters the intervertebral cartilage (Bigner and Johnston, 1981; Takeda et al, 1981). Notochordal cells from nucleus pulposus have been observed in an infant (Takeda et al, 1981). Such cells may mimic a chordoma (see below). Squamous cells and anucleated squames are rare in CSF and are usually of skin origin. Important, but extremely rare sources of squamous cells are a ruptured benign squamous cyst of the brain, a craniopharyngioma, or metastatic squamous carcinoma.
Acellular Components
Corpora amylacea, spherical transparent proteinaceous structures, commonly seen in the brain of the elderly, may occasionally occur in the CSF (Bigner and Johnston, 1981). They are sometimes calcified and mimic a psammoma body or a fungus (Preisig and Buhaug, 1978).
Powder Crystals
Cerebrospinal fluid may be contaminated by starch granules from powder used on surgical gloves. In a personally observed consultation case, the approximately spherical crystals were mistaken for spores of the fungus Cryptococcus neoformans, described below (Fig. 27-1D). Such particles may be phagocytized, as reported by Reinharz et al (1978). In polarized light, the starch particles form a Maltese cross but this may also occur with Cryptococcus. In general, starch particles are larger than Cryptococcus, and often show a central density, but fail to show budding or mucous capsule.
Changes in Benign Cell Populations in Disease
Under pathologic conditions, several changes in the benign cell population of the CSF may take place.
Increase in Cellularity
A mere increase in the number of leukocytes and macrophages always reflects a pathologic process affecting the cerebrospinal fluid. The lesion may be in the brain or in the meninges. Changes in protein and glucose content of CSF often accompany the increased cellularity.
Transformation of Lymphocytes and Monocytes
Under a broad variety of circumstances, such as viral meningitis and most forms of chronic inflammation such as tuberculous meningitis, lymphocytes increase in number and may undergo a transformation that increases substantially the variety of lymphoid cells. These cells range in size from small lymphocytes to large cells, resembling immunoblasts, with a clear nucleus and large nucleolus. The monocytes may be transformed into macrophages and may assume a phagocytic activity (Fig. 27-2A). Oehmichen (1976) characterized mononuclear phagocytes in CSF using membrane markers. Newer methods of classification of mononuclear cells will be described as needed.
Polymorphonuclear Leukocytes
These cells normally do not cross the blood-brain barrier; hence, they must be considered as invaders of the CSF. The presence of neutrophilic polymorphonuclear leukocytes in CSF always indicates an acute inflammatory process, such as bacterial meningitis, brain abscess, some forms of viral encephalitis, or sometimes a reaction to intrathecal chemotherapy. The cells most likely are derived directly from damaged blood capillaries located within the brain or the meninges.
Eosinophils may occur in CSF as a consequence of a parasitic infection of the central nervous system (Cysticercus cellulosae cysts, see below), in chronic inflammations, or as a reaction to trauma. The condition is extremely rare in my experience. Conrad (1986) observed eosinophils in metastatic cancer and in children with a shunt. We have seen such cells in granulocytic sarcoma (see below).
Red Blood Cells and Hemosiderin
Erythrocytes do not normally cross the blood-brain barrier. The most common source of erythrocytes in CSF is a traumatic tap, surgical intervention, trauma, or brain hemorrhage. The presence of hemosiderin-laden macrophages, on the other hand, indicates a previous hemorrhagic event. This may be caused by a cerebral or subdural hemorrhage or the presence of a tumor. Bernad and Taft (1980)
documented that the presence of numerous hemosiderin-bearing macrophages was consistent with an intraventricular hemorrhage in a neonate. In appropriate cases, an iron stain should be performed to differentiate hemosiderin from melanin pigment. Macrophages containing melanin have been observed in melanosis of the meninges (Rosenthal, 1984) that must be differentiated from hemosiderin-bearing cells or cells of primary or metastatic malignant melanoma (see below).
documented that the presence of numerous hemosiderin-bearing macrophages was consistent with an intraventricular hemorrhage in a neonate. In appropriate cases, an iron stain should be performed to differentiate hemosiderin from melanin pigment. Macrophages containing melanin have been observed in melanosis of the meninges (Rosenthal, 1984) that must be differentiated from hemosiderin-bearing cells or cells of primary or metastatic malignant melanoma (see below).
Plasma Cells
The presence of plasma cells in CSF always indicates an important abnormality. This may be a neurologic disorder, a chronic inflammatory event, or plasma cell myeloma (see below).
Cytology in Specific Nonmalignant Disorders
Effects of Myelograms
The injection of a radiopaque medium into the spinal canal results in activation of monocytes into macrophages (Fig. 27-2A). The large cells with round or kidney-shaped nuclei and vacuolated cytoplasm increase in number, become quite conspicuous, and often contain phagocytized deposits of the yellow radiopaque material.
Acute Bacterial Meningitis
Cerebrospinal fluid in meningitis caused by Neisseria meningitidis, Haemophilus influenzae, pneumococci, or other pyogenic organisms, is characterized by the dominance of numerous neutrophilic polymorphonuclear leukocytes. The glucose content of CSF is reduced and the protein level is markedly elevated. Some offending organisms, such as Klebsiella, can sometimes be recognized (Fig. 27-2B).
Viral Meningitis and Meningoencephalitis
Viral (aseptic) meningitis may be caused by a variety of RNA or DNA viruses.
The initial cytologic abnormality, usually of a very short duration (1 or 2 days) is the presence of neutrophilic leukocytes (Kölmel, 1976). The neutrophiles are rapidly replaced by a population of activated lymphocytes and some plasma cells that may vary in numbers but this variability is not indicative of any specific viral type (Fig. 27-2C). Kölmel (1976) reported the highest counts (3,000 cells per mm3) in Coxsackie virus meningitis and the lowest count (40 cells per mm3) in herpes zoster meningitis. As the disease process progresses, there is an increase in monocytes and macrophages (Fig. 27-2D). With healing, the cell count returns to normal.
It is of interest that the sequence of cellular events in CSF (neutrophilic phase to lymphocytic phase to macrophage phase) is similar in all inflammatory afflictions of the meninges, regardless of etiology (see below). The lymphocytic picture of viral meningitis may cause problems in the differential diagnosis of malignant lymphoma and may require a flow cytometric or immunocytochemical analysis of the lymphocytes (Moriarty et al, 1993; Windhagen et al, 1999).
Tick-Borne Encephalitis
This is a serious CNS disorder, prevalent in central and Eastern Europe. The disease is caused by arboviruses, transmitted from wild animals to humans by ticks. Jeren and Vince (1998) studied the make-up of the CSF in this disease by morphologic and immunocytologic analyses. They observed that, during the first 3 days of the illness, there was a prevalence of polymorphonuclear leukocytes, accompanied by a few eosinophiles and basophiles. During the second week of illness, the leukocytes were replaced by lymphocytes and macrophages. Jeren and Vince documented that the prevalent lymphocytes were T-cells (CD3 positive), whereas B-cells (CD20 positive) and macrophages (CD32 positive) were in the minority. Of the T-cells, most were suppressor cells (CD8 positive), with a lesser population of helper cells (CD4 positive).
La Crosse Encephalitis
This sometimes fatal illness occurring in childhood is caused by a mosquito-transmitted virus, first isolated in La Crosse County, Wisconsin (McJunkin et al, 2001). The disease may masquerade as a viral meningitis or herpetic encephalitis and the diagnosis requires identification of the virus. The cerebrospinal fluid shows an increase in lymphocytes in most, but not all, cases and, hence, the cytologic findings are not specific.
Herpes Simplex Encephalitis
Encephalitis caused by infection with herpes simplex virus type I or II is characterized by hemorrhagic necrosis of the brain (review by Crumpacker et al, 2003). Gupta et al (1972) were the first to observe the characteristic virusinduced inclusions in cells in CSF. For the detailed descriptions of the herpes virus-induced cellular changes, see Chapter 10.
Progressive Multifocal Leukoencephalopathy
This disorder, caused by infection with human polyomavirus type JC, is discussed at length in Chapters 22 and 39. This previously rare brain disease has increased markedly in frequency with the onset of AIDS (recent summary in Greenlee, 1998). The characteristic homogeneous basophilic nuclear inclusions in oligodendrocytes and astrocytes have not been reported in CSF. However, the presence of the JC viral DNA may be documented by polymerase chain reaction (PCR) of CSF (Fong et al, 1995). D’Arminio Monforte et al (1997) documented that PCR of CSF is at least equal to brain biopsies in the diagnosis of this essentially incurable disorder.
Tuberculosis
After a lull of several years, tuberculous meningitis is being seen again with increasing frequency, in part because of the AIDS epidemic. The cytologic picture is not specific and depends on the stage of the disease. In early stages, the CSF is very rich in cells, initially neutrophilic leukocytes, followed by transformed lymphocytes, plasma cells, and activated macrophages (see Fig. 27-2D). The changes in cell population are accelerated by therapy (Jeren and Beus, 1982). The presence of multinucleated giant cells has been noted (Kölmel, 1976) but this finding is nonspecific. For example, Bigner et al (1985) observed multinucleated giant cells in CSF as a reaction to foreign material introduced during surgery and in a patient with sarcoidosis.
Fungal Meningitis
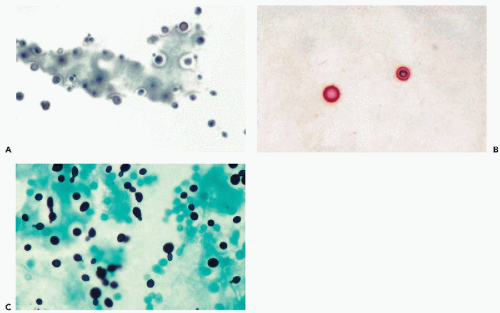
The most common fungus causing meningitis, particularly in immunocompromised or debilitated patients, is Cryptococcus neoformans. This was the most common agent identified in adults in a review of nearly 6,000 spinal fluid samples by Prayson and Fischler (1998). The round or oval yeast organisms, measuring from 4 to 10 μm in diameter, are provided with a thick, mucoid capsule that can be easily visualized by lowering the condenser of the microscope (Fig. 27-3A). The diagnosis can be confirmed by a number of mucus stains [mucicarmine, periodic acid-Schiff (PAS); Fig. 27-3B], by a silver stain (Fig. 27-3C), or an India ink preparation. The organism can also produce single, tearshaped spores and may form hyphae (see Fig. 27-3C, see Chap. 19).
The microscopic diagnosis of the mature organism is comparatively easy, the only possible source of confusion being grains of powder derived from surgical gloves (see Fig. 27-1D). Meningeal cryptococcosis causes only a minimal cytologic reaction in CSF: the organisms are accompanied by a scattering of mature lymphocytes and an occasional macrophage.
In immunocompromised hosts, such as patients with AIDS or patients undergoing intensive chemotherapy, meningitis caused by other fungi can be observed. Thus, Candida albicans (moniliasis), mucormycosis (zygomycosis, phycomycosis), and aspergillosis have been observed. Undoubtedly, other fungi will be observed with the passage of time. For a detailed description of fungi, see Chapters 10 and 19. For toxoplasmosis, see Chapter 39.
Lyme Disease
Lyme disease, an ubiquitous infection with the tick-transmitted spirochete, Borrelia burgdorferi, may cause meningitis. The CSF may show marked hypercellularity with activated lymphocytes in all stages, plasma cells, and macrophages (Benach et al, 1983; Steere et al, 1983; Razavi-Eucha et al, 1987).
Mollaret’s Meningitis
This exceedingly rare form of periodically recurrent aseptic meningitis of unknown etiology has a very characteristic cytologic presentation in CSF (Gledhill et al, 1975; Mollaret, 1977; Lowe, 1982; Chan et al, 2003). During the acute phase, the CSF has a very high and variegated cellular content with numerous polymorphonuclear leukocytes,
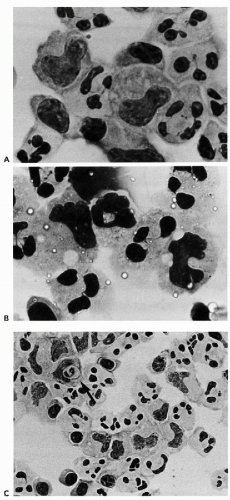
monocytes, plasma cells, and lymphocytes. The dominant cells, however, are large mononucleated (monocytoid) cells with abundant cytoplasm and peculiar nuclei, resembling footprints in the sand (Fig. 27-4A,B). To observe these cells, the fluid should be obtained within 24 hours after onset of the episode of meningitis (Chan et al, 2003). These cells disappear rapidly from the CSF, hence, they are considered to be fragile. Mollaret thought that these cells were possibly of endothelial origin, but their derivation is most likely from transformed monocytes, capable of phagocytosis (see Fig. 27-4C) (Gledhill et al, 1975; Lowe, 1982; Chan et al, 2003). Teot and Sexton (1996) supported the monocyte/macrophage lineage of Mollaret cells by immunocytochemistry. The presence of herpesvirus simplex DNA in this disease has been suggested by polymerase chain reaction (Yamamoto et al, 1991) but could not be confirmed by Teot and Sexton (1996). Chan et al (2003) observed herpesvirus simplex by PCR in 2 of 14 patients.
monocytes, plasma cells, and lymphocytes. The dominant cells, however, are large mononucleated (monocytoid) cells with abundant cytoplasm and peculiar nuclei, resembling footprints in the sand (Fig. 27-4A,B). To observe these cells, the fluid should be obtained within 24 hours after onset of the episode of meningitis (Chan et al, 2003). These cells disappear rapidly from the CSF, hence, they are considered to be fragile. Mollaret thought that these cells were possibly of endothelial origin, but their derivation is most likely from transformed monocytes, capable of phagocytosis (see Fig. 27-4C) (Gledhill et al, 1975; Lowe, 1982; Chan et al, 2003). Teot and Sexton (1996) supported the monocyte/macrophage lineage of Mollaret cells by immunocytochemistry. The presence of herpesvirus simplex DNA in this disease has been suggested by polymerase chain reaction (Yamamoto et al, 1991) but could not be confirmed by Teot and Sexton (1996). Chan et al (2003) observed herpesvirus simplex by PCR in 2 of 14 patients.
Lowe (1982) discussed the differential diagnosis of Mollaret’s meningitis in CSF by citing the findings in a number of very uncommon systemic disorders with incidental meningitis (Behçet’s, Vogt-Koyanagi, and Harada’s syndromes). The reader is referred to specialized sources for further discussion of these very rare events (Hermans et al, 1972).
Other Rare Forms of Meningitis
Sporadic case reports of unusual cytologic findings in CSF in patients with systemic disorders and secondary involvement of the CNS appear from time to time in the literature. Thus, Jaeckle (1982) described the presence of numerous macrophages phagocytosing hemosiderin in a case of systemic lupus erythematosus with transient blindness (Anton’s syndrome). De la Monte et al (1985) observed a polymorphous cell population (transformed lymphocytes, plasma cells, large atypical mononuclear cells) in patients with Sjögren’s syndrome with CNS involvement.
Chemical Meningitis
Chemical meningitis is caused by chemical substances such as chemotherapeutic agents injected into the CSF. Under these circumstances, the CSF is sometimes examined by cytologic methods. In an occasional case, polymorphonuclear leukocytes and activated macrophages can be observed. In a personally observed patient with metastatic mammary carcinoma treated with intrathecal methotrexate, enlargement and cytoplasmic vacuolization were observed in cancer cells in CSF (see below).
Endometriosis
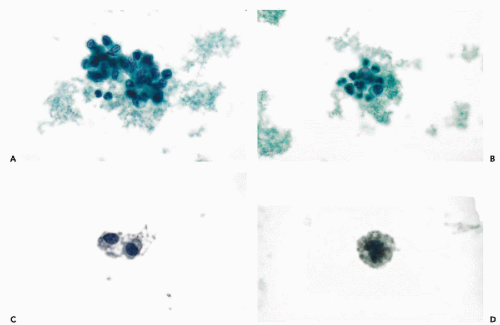
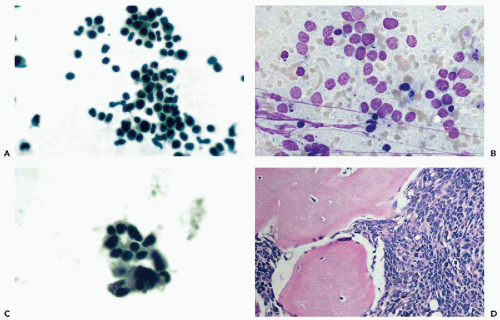
In a personally observed case, periodic headaches synchronous with menstrual bleeding in a 32-year-old woman, were caused by a focus of meningeal endometriosis. In the CSF, clusters of typical endometrial glandular cells were observed. The tap also contained red blood cells and hemosiderin-laden macrophages (Fig. 27-5A,B).
Langerhans’ Cell Histiocytosis
The finding of large mononuclear cells with nuclear grooves (“coffee bean nuclei”), common in this disorder, was reported
in CSF by Ghosal et al (2001). For further comments on this disorder, see Chapters 19, 26, and 31.
in CSF by Ghosal et al (2001). For further comments on this disorder, see Chapters 19, 26, and 31.
Syringomyelia
Courtesy of Dr. Robert Hutter, highly atypical cells of unknown origin or significance were observed in cerebrospinal fluid in a case of syringomyelia, a congenital malformation of the spinal cord with progressive cystic dilatation of central canal. The fluid was presumably aspirated from the central canal (Fig. 27-5C,D).
Neurologic Disorders
Guillain-Barré Syndrome
This is an acute, progressive form of neuropathy of unknown etiology. The CSF is characterized by an increase in proteins without increase in cell population. Nyland and Ness (1978) observed an occasional increase in CSF lymphocytes. We observed a case of meningitis in a patient thought to have a Guillain-Barré syndrome with markedly increased cellularity. The sediment was composed of lymphocytes and numerous macrophages. The possibility that the patient had synchronous meningitis caused by a viral infection could not be ruled out.
Multiple Sclerosis
Multiple sclerosis is a chronic disorder in which loss of myelin occurs in plaques, affecting the white matter in the brain and the spinal cord. The disease, lasting many years and more often affecting young women than men, goes through stages of exacerbation and remissions. The disease is generally considered to be an autoimmune disorder, although the exact pathogenesis has not been determined as yet. There is extensive neurologic literature on the significance of cytologic observations in CSF in multiple sclerosis (summary in Andersson et al, 1994). Zeman et al (2001) described quantitative and qualitative cytologic changes occurring in CSF during the course of the disease. Briefly, the cell counts were only slightly elevated in nearly all patients, regardless of stage of disease. The cell population consisted of activated and inactive lymphocytes and macrophages whereas neutrophilic leukocytes have never been observed. Of particular interest was the presence of “foam cells” or phagocytic macrophages and lipophages, that is, macrophages ingesting fragments of myelin, staining for fat. Also of note was the presence of immature and mature plasma cells. There is no persuasive evidence that the cell counts or cell population is of prognostic value. The counts were lower in treated patients but variations may occur spontaneously in the course of the disease. Fragments of myelinated nerves have been observed by us in CSF from a young woman, during a severe, debilitating relapse of the disease (Fig. 27-6A,B). This appears to be a unique observation that has not been reported before.
Fluid From Ventricular Shunts
Cerebroabdominal shunts are used to alleviate intracranial pressure and reduce hydrocephalus. Bigner et al (1985) described
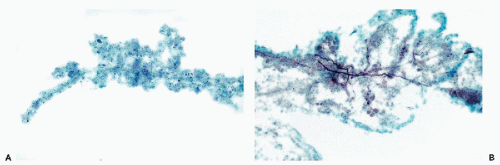
the cytologic finding in the CSF in three patients. Foreign body giant cells, papillary fronds derived from the choroid plexus and, in one case, tumor cells from a malignant pineal germinoma, were observed. In one ascitic fluid sample, fragments of choroid plexus were noted. McCallum et al (1988) also identified cells of the rare choroid plexus carcinoma in ascitic fluid of a 5-year-old child with a shunt. Kimura et al (1984) described peritoneal implants of an endodermal sinus tumor of pineal origin (see Chap. 26).
the cytologic finding in the CSF in three patients. Foreign body giant cells, papillary fronds derived from the choroid plexus and, in one case, tumor cells from a malignant pineal germinoma, were observed. In one ascitic fluid sample, fragments of choroid plexus were noted. McCallum et al (1988) also identified cells of the rare choroid plexus carcinoma in ascitic fluid of a 5-year-old child with a shunt. Kimura et al (1984) described peritoneal implants of an endodermal sinus tumor of pineal origin (see Chap. 26).
Cancer Cells
An obvious condition for a successful cytologic diagnosis of a primary or metastatic malignant tumor is the seeding of tumor cells into the subarachnoid space or into the
cerebral ventricles. Even in lesions that are in contact with CSF, the number of malignant cells observed may be very small; the diagnosis may have to rest on the presence of a dozen, or sometimes fewer, malignant cells. However, in this particular setting, even a single abnormal cell should be most carefully evaluated as it may prove to be of diagnostic value. The sources of error are relatively few and rarely of importance.
cerebral ventricles. Even in lesions that are in contact with CSF, the number of malignant cells observed may be very small; the diagnosis may have to rest on the presence of a dozen, or sometimes fewer, malignant cells. However, in this particular setting, even a single abnormal cell should be most carefully evaluated as it may prove to be of diagnostic value. The sources of error are relatively few and rarely of importance.
Only in malignant lymphomas and leukemias and the exceedingly rare primary meningeal sarcomas are abundant cancer cells consistently present in CSF. In other malignant tumors, the presence of abundant cancer cells in CSF almost invariably indicates an extensive involvement of leptomeninges (meningeal carcinomatosis).
Primary Tumors of the Central Nervous System
For description, classification, and cytologic presentation of tumors of the central nervous system, see Chapter 39. Few of the primary brain tumors involve or spread to the meninges, except medulloblastoma and related tumors of childhood (see below). On the rarest occasions, high-grade astrocytomas may metastasize to the meninges and the extracranial lymph nodes. Pasquier et al (1980) described two such cases and summarized more than 70 similar cases from the literature. Additional examples were cited and illustrated by Kölmel (1976).
Thus, the CSF is rarely studied as a means of diagnosis of primary brain tumors in adults and the cytologic observations may be considered incidental. In fact, much of the writing on this subject is based on touch preparations of biopsy fragments or on CSF obtained after surgery (Watson and Hajdu, 1977). Naylor’s data (1964) also suggest that the cerebrospinal fluid, obtained directly from cerebral ventricles or the cisterna magna, is a better medium of diagnosis than spinal tap. Nonetheless, occasionally an unexpected diagnosis of a primary brain tumor can be suggested (or confirmed) on cytologic examination of the CSF and this is the purpose of this brief synopsis. Several major reviews of this subject have been published by Kline (1962), Naylor (1964), Gondos and King (1967), Watson and Hajdu (1977), Bigner and Johnston (1981), Ehya et al (1981), and Rosenthal (1984).
The recognition of cells originating in the primary neoplasms of the CNS in CSF depends on two factors: anatomic location and degree of differentiation. Tumors located within the depths of the brain or the spinal cord, and thus not bathed by CSF, cannot be recognized except by direct sampling. Well-differentiated tumors (astrocytomas grade I and II, or choroid plexus tumors) cannot be recognized in spinal fluid, except as “unusual” or “foreign” cells (i.e., cells that are not normally observed). For example, cells from ependymomas usually appear as benign cuboidal or columnar cells. Tumors that shed recognizable cancer cells (such as astrocytomas of high-grade, glioblastoma multiforme, medulloblastomas and related tumors, or malignant tumors of the pineal) may be occasionally recognized in CSF. The cytologic presentation of the most common primary tumors of the CNS in CSF follows.
Low-Grade Astrocytomas (Grades I and II)
These tumors of glia shed very few, if any, cells into the CSF and we have not seen an example of it. According to the published reports, the tumor cells are spindly, although occasionally, stellate cytoplasm may be observed. The nuclei are pale, not enlarged, and cannot be recognized as malignant. In fact, Bigner and Johnston (1981) pointed out that identical cells may be observed in benign destructive processes of the brain. Watson and Hajdu (1977) recognized only one such tumor in the CSF.
High-Grade Astrocytomas and Glioblastoma Multiforme
The CSF may contain a few abnormal cells with nuclear enlargement and hyperchromasia. In the absence of clinical history, an accurate diagnosis is unlikely. In one of the personal cases of glioblastoma multiforme, the CSF contained a moderate number of large cells, some with eosinophilic and some with transparent cytoplasm and large single nuclei with prominent nucleoli (Fig. 27-7A). The appearance of the cells was suggestive of a metastatic epithelial tumor rather than a malignant glioma. However, a review of the corresponding tissue sections suggested that these cells were most likely abnormal astrocytes (Fig. 27-7B). Multinucleated giant tumor cells, so characteristic of this tumor in tissue, were not seen by us in CSF. When observed, they are strongly suggestive of this neoplasm (Kölmel, 1974). The diagnosis of a primary high-grade brain tumor in CSF should be made only in the presence of confirmatory clinical and roentgenologic evidence. An important caveat is in order here: the presence of numerous conspicuous cancer cells in CSF is much more likely to represent a metastatic tumor than a high-grade glioma.
Oligodendroglioma
Watson and Hajdu (1977) described the cytology of this tumor in a touch preparation as made up of uniformly round cells with round and eccentric nuclei provided with nucleoli. This is the appearance of these cells in touch preparations seen in our laboratories (see Chap. 39).
Ependymomas
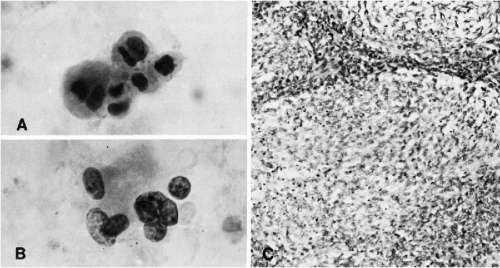
Ependymomas, and their cytologic presentation in direct brain samples, are discussed in Chapter 39. These tumors may occasionally shed cells in the CSF. In a personally observed case of recurrent “cellular ependymoma” in a 19-year-old man, the CSF contained small cells with relatively large hyperchromatic nuclei, occurring singly or in small clusters (Fig. 27-7C,D). None of the characteristic features of ependymoma (cuboidal or columnar cells in palisade-like arrangement or rosette formation) were observed in this case.
Midline Tumors
For a discussion of histology and cytology of this group of tumors, see Chapter 39. Only germinomas can be recognized
in CSF. Thus, Ginhorst and Tskahara (1979) described a case of recurrent pineal germinoma with pulmonary metastases in which large tumor cells were recognized in CSF and in sputum. Of note was the elevation of the beta subunit of human chorionic gonadotropin in CSF. Several additional cases of germinomas with cells in CSF have been described (Zaharopoulos and Wong, 1980; Bigner and Johnston, 1981; Geisinger et al, 1984). In patients with germinomas and other highly malignant tumors treated with an abdominal shunt, the cancer cells may also be recognized in ascitic fluid (see Chap. 26).
in CSF. Thus, Ginhorst and Tskahara (1979) described a case of recurrent pineal germinoma with pulmonary metastases in which large tumor cells were recognized in CSF and in sputum. Of note was the elevation of the beta subunit of human chorionic gonadotropin in CSF. Several additional cases of germinomas with cells in CSF have been described (Zaharopoulos and Wong, 1980; Bigner and Johnston, 1981; Geisinger et al, 1984). In patients with germinomas and other highly malignant tumors treated with an abdominal shunt, the cancer cells may also be recognized in ascitic fluid (see Chap. 26).
Medulloblastomas and Related Tumors
Medulloblastomas occur mainly in the cerebellum of children and young adults. The tumors are related and morphologically similar to neuroblastomas and retinoblastomas, are highly malignant and consistently spread to the meninges, and may also form distant metastases (see Fig. 27-8D). After leukemia, this is the most common malignant tumor of childhood that can be recognized in CSF (Prayson and Fischler, 1998). For further comments on the structure of medulloblastomas, see Chapter 39.
Cytology
In CSF, the cells of medulloblastoma are usually numerous and readily identified as malignant. The cells vary in size. They are larger in some tumors than in others, but within each tumor, remain fairly monotonous (Fig. 27-8A,B). The cancer cells may occur singly, in small clusters, and may form rosettes (Fig. 27-8C). The cytoplasm is scanty, readily visible, and is sometimes elongated. The nuclei are hyperchromatic. Prominent nucleoli are clearly visible in well-fixed and well-stained material. Such cancer cells cannot be distinguished from cells of metastatic retinoblastoma (Fig. 27-9A,B), or embryonal carcinoma, and when occurring singly, may mimic a malignant lymphoma (see below). Still, in fortuitous cases, cells of neuroblastoma may show cytoplasmic filaments (see Fig. 27-9A).
Choroid Plexus Tumors
The rare choroid plexus papillomas and carcinomas may also be identified in CSF. The papillomas sometimes shed cohesive clusters of epithelial cells in papillary arrangements. The cells of carcinomas have the features of any adenocarcinoma, with large nuclei and prominent, large nucleoli (Bigner and Johnston, 1981; Kim et al, 1985). As always, when confronted with large cancer cells in CSF, the differential diagnosis with metastatic cancer must be considered. Clinical and roentgenologic data are essential in the interpretation.
Spindle Cell Sarcomas
Spindle cell sarcomas of the brain are very rare, difficult to classify, and highly malignant. We have observed one such case with obvious cancer cells in CSF in a patient with a recurrent tumor (Fig. 27-10). The cancer cells had no distinguishing features and could have been mistaken for cells of metastatic carcinoma because of abundant cytoplasm.
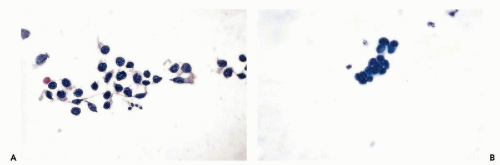 Figure 27-9 Neuroblastoma (A) and retinoblastoma (B). Both tumors show nearly identical small tumor cells in CSF. Note cytoplasmic filaments in A. |
Primary Malignant Lymphomas of the Brain
These tumors are seen with increased frequency in AIDS patients (see Chap. 39). The cytologic presentation of lymphomas is described below.
Squamous Carcinoma
A case of primary intracranial squamous carcinoma derived from an epidermoid cyst at the base of the brain, was described by Bondeson and Falt (1984), who observed keratinized cancer cells in CSF. Cells of a ruptured craniopharyngioma resemble squamous cancer cells (see Chap. 39). See comment on metastatic squamous carcinoma below.
TABLE 27-1 CEREBROSPINAL FLUID: DIAGNOSTIC RESULTS WITH SOME PRIMARY TUMORS OF THE CENTRAL NERVOUS SYSTEM EXPRESSED AS PERCENTAGE OF POSITIVE RESULTS | |
|---|---|
|