Central Nervous System
Jack Raisanen
Charles L. White III
Intraoperative Diagnosis in Neuropathology
Intraoperative diagnosis often plays a role in the care of the patient with an intracranial or intraspinal mass. The reason for intraoperative diagnosis is to help the surgeon determine the endpoint of the operation. In some instances, clinical presentation and preoperative imaging lead to a biopsy as the initial surgical procedure and, in this setting, the pathologist is asked to examine a portion of the specimen to be certain that diagnostic tissue has been obtained. In other cases, a mass is widely exposed and an intraoperative decision about the extent of resection is based on the diagnosis and the surgeon’s ability to identify an interface between the abnormality and adjacent structures.
Intraoperative diagnosis may hasten postoperative care of the patient by leading to immediate body scans in cases of metastatic disease or, unusually, immediate treatment of life-threatening herniation. Finally, intraoperative diagnosis helps to determine the need for additional unfixed tissue for studies to help to characterize a neoplasm or for culture to identify microorganisms.
There are a wide variety of diseases, including many types of neoplasms, that affect the central nervous system (CNS), and it may not be possible to render an accurate intraoperative diagnosis on morphologic features alone. It is therefore best to make the diagnosis in the context of the patient’s clinical presentation. Information that is key to successful intraoperative diagnosis includes the age of the patient, the location of the lesion and its radiographic appearance. For instance, radiographs, particularly magnetic resonance images, may indicate the demarcation of a neoplasm, whether it is cystic, surrounded by edema, causes mass effects, or exhibits contrast enhancement.
A number of excellent references are available to the pathologist who consults intraoperatively in neuropathology (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19); we have drawn some of the information for this chapter from those references.
Intraoperative Diagnosis During Resection
A neurosurgeon who is operating with wide exposure with intent to resect a lesion in its entirety will often submit a small fragment of the lesion for intraoperative diagnosis. The lesion may be contained in, or be submitted
along with, normal CNS parenchyma. Unfixed normal CNS gray matter is reddish brown and normal white matter is white or near-white. Lesional tissue often can be identified in normal parenchyma by finding areas of hemorrhage or necrosis or, sometimes, in cases of diffusely infiltrating gliomas, areas that are yellowish or gray, or softer than the surrounding parenchyma. It is also possible to locate a diffusely infiltrating glioma by identifying areas where the normally sharp gray/white matter junction is effaced.
along with, normal CNS parenchyma. Unfixed normal CNS gray matter is reddish brown and normal white matter is white or near-white. Lesional tissue often can be identified in normal parenchyma by finding areas of hemorrhage or necrosis or, sometimes, in cases of diffusely infiltrating gliomas, areas that are yellowish or gray, or softer than the surrounding parenchyma. It is also possible to locate a diffusely infiltrating glioma by identifying areas where the normally sharp gray/white matter junction is effaced.
It is best to divide the submitted tissue three ways: Tissue for frozen section, tissue for a cytologic preparation (both for intraoperative diagnosis), and nonfrozen tissue for formalin fixation for final diagnosis. In some cases, depending upon the intraoperative diagnosis, it may be helpful to further divide the tissue set aside for final diagnosis and to put fragments in glutaraldehyde for electron microscopy or in media for flow cytometric or cytogenetic studies. If cultures are indicated, it is preferable that they be obtained in the operating room rather than the surgical pathology lab.
For intraoperative cytology, we prefer smear preparations instead of touch preparations because of increased cellularity. We use approximately 1 mm3 of tissue and simultaneously crush and smear it between two glass slides. We fix the smear in 100% methanol and stain it with hematoxylin and eosin.
Intraoperative Diagnosis during Biopsy
A neurosurgeon who is operating to biopsy a lesion wants to know if he or she has submitted tissue that will lead to a diagnosis upon which appropriate treatment can be based. Usually, the biopsy is done with a needle with a hollow core, and small, cylindrical fragments are submitted. Once again, the lesion may be submitted in or along with normal CNS parenchyma and it may be necessary to identify areas that are necrotic, hemorrhagic, yellowish gray, or gelatinous.
Intraoperative diagnosis of a neoplasm, an infection, or, possibly, features consistent with an infarct or focus of demyelination indicates to the surgeon that he or she has sampled the lesion. It is best, however, if the neurosurgeon stops the procedure only if sufficient tissue has been or will be obtained for formalin fixation, culture, or other studies necessary to confirm the intraoperative impression.
Non-Neoplastic Lesions
Some of the most important intraoperative diagnoses in neurosurgery are diagnoses of non-neoplastic lesions, because patients with such lesions may not benefit from resection. Thus, the first thing to consider when examining a CNS lesion during a planned resection is whether the pathologic findings represent a destructive, but non-neoplastic process; specifically, does the increased cellularity represent hyperplasia and not neoplasia? The most common destructive, but non-neoplastic, lesions that mimic neoplasms clinically and radiologically are (i) infections/abscesses, (ii) infarcts, and (iii) plaques of demyelinating diseases like multiple sclerosis.
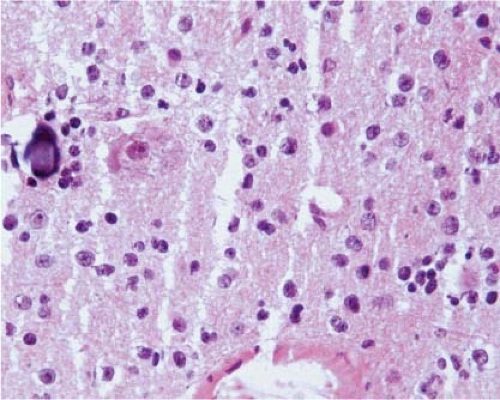
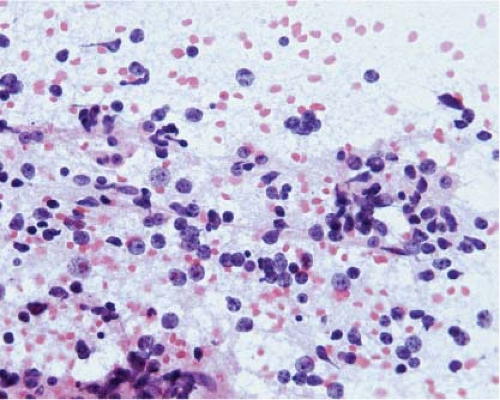
At the time they are most likely to mimic neoplasms, infarcts are usually organizing and the parenchyma contains inflammatory cells, usually abundant foamy macrophages, and reactive astrocytes. Similarly, at the time demyelination mimics a neoplasm, there is ongoing destruction and the plaque contains lymphocytes, many foamy macrophages, and reactive astrocytes. Thus, the key to identification of these two lesions intraoperatively is recognition of foamy macrophages and reactive astrocytes (20) (Fig. 15.1). Frozen sections of parenchyma infiltrated by foamy macrophages can be particularly confusing because the cell borders of macrophages are often difficult to distinguish, their nuclei are monomorphic and resemble those of oligodendrocytes, and their clear cytoplasm resembles ice crystal artifact. In these cases, cytologic preparations that reveal individual cells are very useful. They show cells with monomorphic nuclei with diameters that are approximately 1.5 to 2 times that of normal oligodendrocytes, abundant foamy or granular cytoplasm, and low nuclear-to-cytoplasmic (N/C) ratios (Fig. 15.2, e-Fig. 15.1). Sections and smears show reactive astrocytes with low N/C ratios; abundant perinuclear cytoplasm; and multiple long, highly fibrillar processes (e-Fig. 15.2). In both organizing infarcts and active plaques of demyelination, reactive astrocytes are greatly outnumbered by foamy macrophages.
The observation of necrotic neuronal cell bodies suggests infarct instead of demyelination, but they are often hard to identify on intraoperative
preparations, and the distinction must be made using formalin-fixed, paraffin-embedded sections and, sometimes, special stains.
preparations, and the distinction must be made using formalin-fixed, paraffin-embedded sections and, sometimes, special stains.
It is ultimately up to the surgeon to correlate the intraoperative diagnosis of inflammation and astrocytosis with his or her impression of the lesion. As neurosurgeons reach an organizing infarct or a plaque of demyelination,
they often suspect that the lesion is non-neoplastic and, in these cases, an intraoperative diagnosis of inflammation with abundant foamy macrophages and reactive astrocytosis will usually lead them to abandon a plan for complete resection. However, the presence of reactive astrocytes and infiltrates of inflammatory cells alone merely indicates tissue injury and may be observed in the parenchyma adjacent to a neoplasm; thus, the surgeon who suspects a neoplasm must persist in obtaining additional samples for microscopic examination.
they often suspect that the lesion is non-neoplastic and, in these cases, an intraoperative diagnosis of inflammation with abundant foamy macrophages and reactive astrocytosis will usually lead them to abandon a plan for complete resection. However, the presence of reactive astrocytes and infiltrates of inflammatory cells alone merely indicates tissue injury and may be observed in the parenchyma adjacent to a neoplasm; thus, the surgeon who suspects a neoplasm must persist in obtaining additional samples for microscopic examination.
Infection of the CNS parenchyma by bacteria, fungi, or parasites often leads to an infiltrate composed of combinations of neutrophils, lymphocytes, plasma cells, and macrophages. Macrophages may be foamy, epithelioid, or multinucleated, and there are usually nearby reactive astrocytes. As with infarcts and demyelinating plaques, neurosurgeons often recognize an abscess as they approach it, and intraoperative diagnosis is used to confirm their impression. Surgeons may then turn their attention to draining or resecting an abscess and sending tissue to the microbiology laboratory for identification of microorganisms. Sometimes it is possible to identify organisms intraoperatively on sections or smears, for instance, fungi or Toxoplasma.
Neoplasms
Many types of neoplasms arise in the CNS, impinge on it, or are metastatic to it, and, as in other systems, specific types occur most frequently in specific locations in specific age groups. Because of this, it is best to make the diagnosis of a specific type of neoplasm in the context of the patient’s age and the location of the neoplasm. With regard to location, it is useful to divide the CNS into supratentorial, infratentorial, and spinal compartments, and to consider whether a supratentorial or infratentorial neoplasm is intraventricular, intraparenchymal (intra-axial), or extraparenchymal (extra-axial), and whether a spinal neoplasm is intraparenchymal (intramedullary), extraparenchymal but intradural, or epidural. Supratentorial neoplasms may be localized further to the sellar or pineal regions. In addition, some cerebral parenchymal neoplasms extend into the third or lateral ventricles and some sellar or pineal region neoplasms are found mostly in the third ventricle. Tables 15.1 and 15.2 list the most common neoplasms in these specific CNS locations in adults and children.
The intraoperative diagnosis of a specific neoplasm helps the neurosurgeon determine the extent of the resection, a decision based in part on (i) the optimal treatment for the neoplasm and (ii) the known, usual biologic behavior of the neoplasm. For instance, some CNS neoplasms, like lymphoma, are best treated medically instead of surgically. For most neoplasms, though, surgery is the best initial treatment and surgeons want to know the characteristics of the border between neoplasm and normal parenchyma. Specifically, individual cells of some CNS neoplasms are able to infiltrate normal parenchyma diffusely, while other neoplasms are
composed of cells that do not infiltrate aggressively and are relatively well circumscribed. In this regard, metastatic neoplasms may be relatively well circumscribed, but are often multifocal.
composed of cells that do not infiltrate aggressively and are relatively well circumscribed. In this regard, metastatic neoplasms may be relatively well circumscribed, but are often multifocal.
Table 15.1 Most Common CNS Neoplasms in Adults | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Sometimes, even with clinical and radiographic information, it is not possible to name the specific type and grade of a neoplasm intraoperatively, either because of artifact, because the amount of tissue is too small, or because immunohistochemistry or other studies are necessary. In these instances, the most helpful intraoperative diagnosis describes the likely biologic behavior of the neoplasm, particularly, the border of the neoplasm with normal tissue. Thus, the intraoperative diagnosis of “low-grade diffusely infiltrating glioma” states that the specific differentiation of the neoplasm is uncertain, but indicates that all of the neoplastic cells probably cannot be resected. Sometimes, when type is uncertain, it is helpful to clarify a prediction of likely biologic behavior with a diagnosis that includes the differential. For instance, an intraoperative diagnosis for a
posterior fossa neoplasm in an adult may be “low-grade spindle cell neoplasm: Schwannoma versus fibrous meningioma.”
posterior fossa neoplasm in an adult may be “low-grade spindle cell neoplasm: Schwannoma versus fibrous meningioma.”
Table 15.2 Most Common CNS Neoplasms in Children | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Supratentorial Neoplasms of the Cerebrum and Associated Meninges: Adults
In adults, the most common primary neoplasms of the cerebral parenchyma are diffusely infiltrating gliomas, and the most common metastatic neoplasms are carcinomas. Melanomas, usually metastatic, and lymphomas, either primary or metastatic, are also encountered intraoperatively, but less frequently. The most common supratentorial, extra-axial, but intradural neoplasms are meningiomas.
Diffusely Infiltrating Astrocytoma
Diffusely infiltrating astrocytomas are neoplasms composed of incompletely differentiated astrocytic cells. According to the 2007 World Health Organization (WHO) Classification of Tumours of the Central Nervous System (18), diffusely infiltrating astrocytomas are graded as 2, 3, or 4, with grade 3 synonymous with anaplastic astrocytoma and grade 4 with glioblastoma. The peak incidence of grade 2 neoplasms is between age 30 and 40 and of grade 4 neoplasms between age 45 and 75. Individual cells of diffusely infiltrating astrocytomas invade surrounding normal CNS parenchyma and, therefore, the intraoperative diagnosis of an infiltrating glioma indicates to the surgeon that all of the neoplastic cells probably cannot be resected.
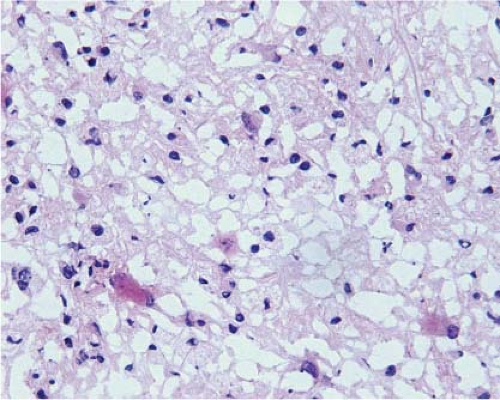
Because of edema, frozen sections of low-grade diffusely infiltrating astrocytomas almost always exhibit severe artifact (Fig. 15.3, e-Fig. 15.3) and it is usually difficult to be certain of nuclear size, atypia, and pleomorphism or of cytoplasmic differentiation. Moreover, cellularity is usually low and, in fact, it is often difficult to be certain of the diagnosis of neoplasm. However, the neoplastic cells infiltrate among axons and normal glial cells, so comparison of neoplastic nuclei with normal glial nuclei is often the key to intraoperative diagnosis, and this is usually easiest on cytologic preparations (Fig. 15.4). Normal oligodendrocyte nuclei are round or almost round with diameters of approximately 8 μm, smooth nuclear membranes and, usually, densely packed chromatin. Normal astrocytes have round to oval or elliptical nuclei with smooth membranes, observable parachromatin spaces and minor diameters of approximately 10 μm. Nuclei of grade 2 diffusely infiltrating astrocytomas have shapes that are similar to those of normal astrocytes, but are larger with diameters that are approximately 1.5 to 2.5 times those of normal glial nuclei and, usually, at least some have atypical membranes. Few mitotic figures may be observed. Some cells may have scant perinuclear cytoplasm, but fibrillar processes are difficult to associate with neoplastic nuclei.
Low-grade oligodendrogliomas are usually composed of cells with uniformly round or almost round nuclei that are neither as atypical nor as pleomorphic as those of low-grade diffusely infiltrating astrocytomas.
However, it is sometimes difficult, even with excellent smears, to distinguish a low-grade diffusely infiltrating astrocytoma from a low-grade oligodendroglioma or a low-grade oligoastrocytoma intraoperatively, and in those cases, “low-grade infiltrating glioma” is an appropriate diagnosis.
However, it is sometimes difficult, even with excellent smears, to distinguish a low-grade diffusely infiltrating astrocytoma from a low-grade oligodendroglioma or a low-grade oligoastrocytoma intraoperatively, and in those cases, “low-grade infiltrating glioma” is an appropriate diagnosis.
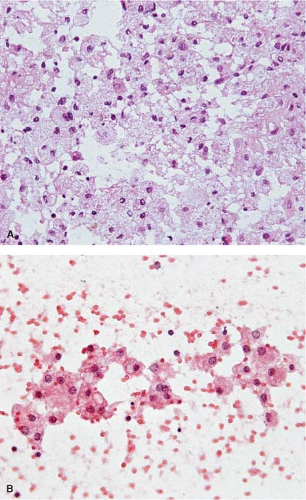
The cells of high-grade diffusely infiltrating astrocytomas have atypical and pleomorphic nuclei usually with coarsely granular, irregularly dispersed chromatin; sometimes, the chromatin is clumped or appears smudged (Fig. 15.5). Some cells contain mitotic figures. Usually, some of the cells have relatively abundant perinuclear cytoplasm and many have long, stellate processes characteristic of glial differentiation. Glioblastomas have tumor cell necrosis or contain microvascular proliferation or both. On frozen sections of a high-grade cerebral neoplasm it may be difficult to distinguish between glioblastoma, carcinoma, melanoma, or lymphoma. The finding of individual neoplastic cells infiltrating normal parenchyma suggests glioblastoma or lymphoma, while a sharp interface suggests carcinoma or melanoma. Sometimes, though, the central portion of a high-grade neoplasm is submitted so that the border of the neoplasm with normal parenchyma is not observable. In these cases, cytologic preparations that reveal cytoplasmic margins are key to distinguishing a high-grade astrocytoma from carcinoma, melanoma, or lymphoma (Fig. 15.6, e-Fig. 15.4).
Oligodendroglioma
Oligodendrogliomas are diffusely infiltrating gliomas composed of cells that resemble normal oligodendrocytes. As with
diffusely infiltrating astrocytomas, individual cells invade surrounding normal parenchyma and, therefore, an intraoperative diagnosis of oligodendroglioma indicates to the surgeon that all of the neoplastic cells probably cannot be resected.
diffusely infiltrating astrocytomas, individual cells invade surrounding normal parenchyma and, therefore, an intraoperative diagnosis of oligodendroglioma indicates to the surgeon that all of the neoplastic cells probably cannot be resected.
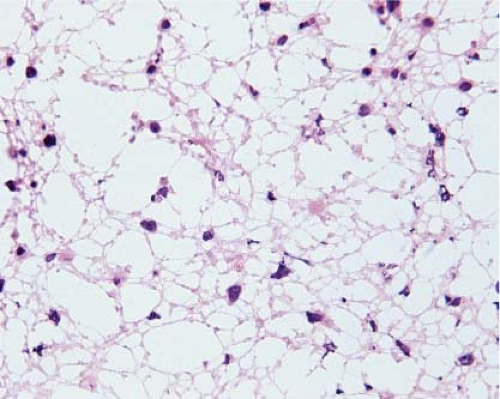
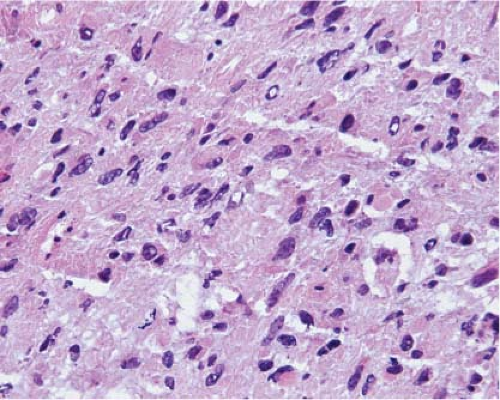 Figure 15.5 Frozen section of a high-grade diffusely infiltrating astrocytoma. The small, round nuclei with densely packed chromatin are the nuclei of normal oligodendrocytes. |
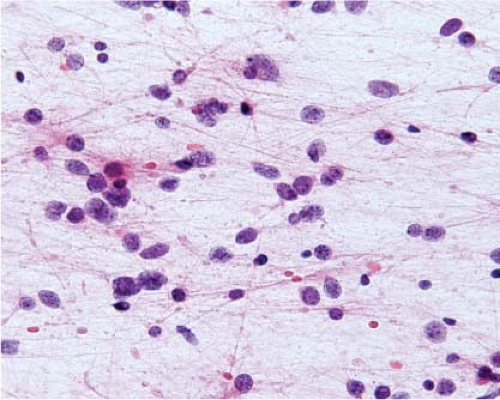
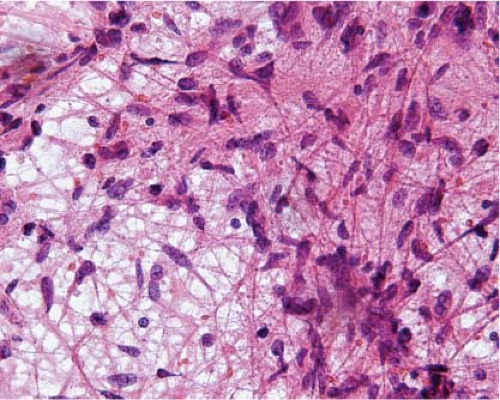 Figure 15.6 Intraoperative smear of glioblastoma. The overlapping, intersecting cytoplasmic processes indicate glial differentiation. |
According to WHO (18), oligodendrogliomas are grade 2 or grade 3; peak incidence of grade 2 neoplasms is between age 40 and 45 years. Low-grade oligodendrogliomas are often composed of round to oval cells without discernible cytoplasmic processes, though in some neoplasms, some cells have short processes. Nuclei are consistently round or nearly round, usually with diameters that are approximately 1.5 to 2.5 times that of normal oligodendrocytes. Chromatin is finely granular, often with one or several larger chromocenters. Sometimes, parachromatin spaces are relatively wide, creating a salt and pepper appearance. Like low-grade infiltrating astrocytomas, the cells are often arranged diffusely among normal axons and glia. Sometimes, they occur in sheets or nodules.
Grade 3 oligodendrogliomas have relatively abundant mitotic figures and/or microvascular proliferation or tumor cell necrosis, or both.
In formalin-fixed, paraffin-embedded sections, the cytoplasm of the neoplastic cells of low-grade oligodendrogliomas often appears clear, but on frozen sections, this artifact is usually missing (Fig. 15.7, e-Fig. 15.5) and, because of low cellularity and other artifacts, diagnosis may be difficult. Therefore, as with low-grade diffusely infiltrating astrocytomas, intraoperative diagnosis often depends on comparison of
normal and neoplastic oligodendroglial nuclei with identification of separate populations, and this is often easiest on smear preparations (Fig. 15.8, e-Fig. 15.6).
normal and neoplastic oligodendroglial nuclei with identification of separate populations, and this is often easiest on smear preparations (Fig. 15.8, e-Fig. 15.6).
Again, it is usually difficult to distinguish low-grade oligodendrogliomas from low-grade diffusely infiltrating astrocytomas or low-grade oligoastrocytomas intraoperatively, because of the degree of overlap of nuclear and cytoplasmic features. Findings that suggest oligodendroglioma or a component of oligodendroglial differentiation include uniformly round, or almost round, nuclei with little atypia or pleomorphism. The intraoperative diagnosis of “low-grade infiltrating glioma” describes the biologic behavior of all three of those neoplasms.
It is also typically difficult to distinguish high-grade oligodendrogliomas from high-grade astrocytomas intraoperatively, thus warranting a diagnosis of “high-grade infiltrating glioma.”
Metastatic Neoplasms
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree