CHAPTER 28 Cells of the Extracellular Matrix and Immune System
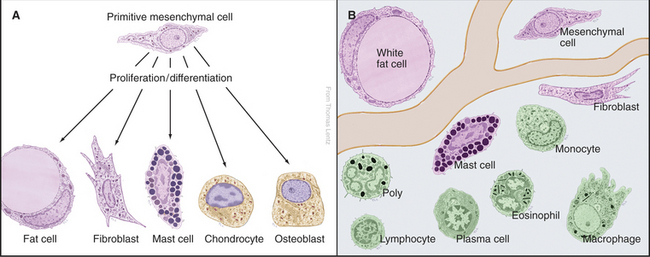
A remarkable variety of specialized cells populate the connective tissues of animals. These cells manufacture extracellular matrix, defend against infection, and maintain energy stores in the form of lipid (Fig. 28-1). Some of these cells arise in connective tissue and remain there. These indigenous cells are specialized: Fibroblasts make the collagen, elastic fibers, and proteoglycans of the extracellular matrix; chondrocytes secrete the matrix for cartilage; and osteoblasts manufacture the calcified matrix of bone. Fat cells store lipids, and mast cells secrete histamine and other mediators of inflammation. The remaining cells arise elsewhere, travel through blood and lymph, and enter connective tissue as needed, so they are known as immigrant cells. These visitors are part of the immune system, which defends against microorganisms. This chapter introduces all of these cells.
Indigenous Connective Tissue Cells
Primitive Mesenchymal Cells
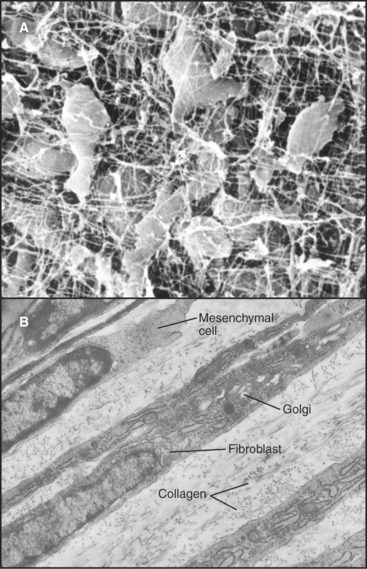
Primitive mesenchymal cells are undifferentiated, multipotential stem cells (see Box 41-1) that proliferate and differentiate (Fig. 28-1) to give rise to all the indigenous cells of connective tissue (fibroblasts, fat cells, mast cells, chondrocytes, and osteoblasts). Small numbers of these inconspicuous precursor cells hide along the small blood vessels but cannot be identified by light microscopy. By electron microscopy (Fig. 28-2), mesenchymal cells resemble fibroblasts but with fewer organelles of the secretory pathway.
Fibroblasts
Fibroblasts are the connective tissue workhorses, synthesizing and secreting most of the macromolecules of the extracellular matrix (Fig. 28-2). Chapter 29 considers the synthesis of these matrix molecules in detail. Accordingly, mature fibroblasts have abundant rough endoplasmic reticulum and a large Golgi apparatus. They are generally spindle-shaped, with an oval, flattened nucleus, but can assume many other shapes depending on the mechanical forces in the surrounding matrix. The migratory patterns of the fibroblasts determine the patterns of collagen fibrils in tissues. In response to tissue damage, fibroblasts proliferate and migrate into the wound, where they synthesize new matrix to restore the integrity of the tissue (see Fig. 32-11).
Mast Cells
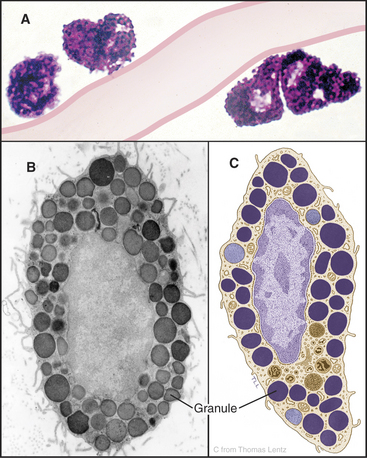
Mast cells are secretory cells that mediate “immediate hypersensitivity” reactions by secreting the contents of histamine-containing granules in response to insect bites or exposure to allergens, as in hay fever. Mast cells distribute along blood vessels in connective tissue (Fig. 28-3). The large, abundant granules contain, by mass, 30% heparin–basic protein complex, 10% histamine, and 35% basic proteins, including proteases. A variety of stimuli can induce secretion of the granule contents. The most specific stimulus operates through plasma membrane receptors for immunoglobulins of the immunoglobulin E (IgE) class. These receptors bind a random selection of soluble IgEs that the immune system makes in response to exposure to allergens. When the corresponding antigen binds to IgE on the surface of a mast cell, the receptors aggregate, triggering a cytoplasmic Ca2+ pulse (see Chapter 26) and fusion of granules with the plasma membrane (see Fig. 21-12). Mechanical trauma, radiant energy (heat, X-rays), and toxins or venoms are less specific stimuli but can also trigger secretion. Outside the cell, the carrier proteins release heparin and histamine. Histamine binds to cellular receptors, causing blood vessels to leak plasma, smooth muscle to contract, and itching sensations to occur. This results in the congestion and constriction of the respiratory tract in allergic reactions and swelling of the skin after an insect bite. Secreted fibrinolysin and heparin inhibit blood clotting. Stimulated mast cells also secrete tumor necrosis factor-a and eicosanoids, contributing to the activation of other inflammatory cells in chronic conditions including asthma and arthritis.
White Fat Cells
Fat cells (adipocytes) distributed in the connective tissue beneath the skin and in the abdominal mesentery of vertebrates store lipids as a readily accessible reserve of energy. These round cells vary in diameter depending on the size of their single, large, lipid droplet (Fig. 28-4) containing triglycerides, neutral lipids with a fatty acid esterified to all three carbons of glycerol (see Fig. 7-2 for the structures of glycerol and fatty acids). Intermediate filaments and endoplasmic reticulum separate the lipid droplet from the thin rim of cytoplasm. After a meal, fat cells take up fatty acids and glycerol from blood and synthesize triglycerides for storage. During fasting or when the body requires energy, enzymes called lipases hydrolyze fatty acids from triglycerides for release back into the blood for use by other organs. Hormones including epinephrine (see Fig. 27-3) regulate these metabolic reactions.
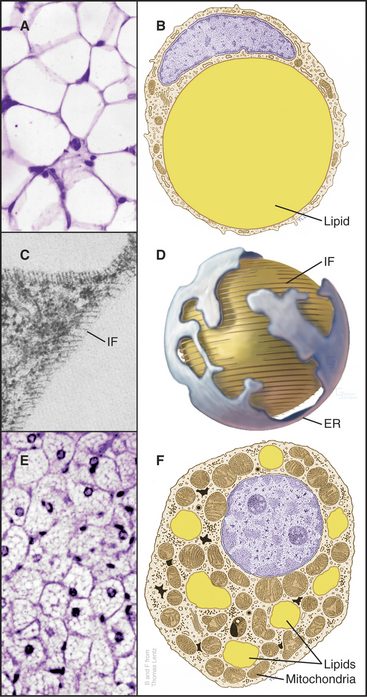
Figure 28-4 fat cells. A., Light micrograph of a section of white fat cells stained with hematoxylin and eosin. B, Drawing of a white adipose cell. C, Transmission electron micrograph of a thin section of the edge of a lipid droplet showing the circumferential sheath of vimentin intermediate filaments (IF [see Chapter 35). D, Interpretive drawing of a lipid droplet with its associated filaments and endoplasmic reticulum (ER). E, Light micrograph of a section of brown fat. F, Drawing of a brown fat cell.
(A, C, and E, Courtesy of D. W. Fawcett, Harvard Medical School, Boston, Massachusetts. B and F, Modified from T. Lentz, Yale Medical School, New Haven, Connecticut. D, Modified from Werner Franke, University of Heidelberg, Germany.)
Mutations in four different genes cause inherited lipodystrophies, human conditions with loss of fat tissue. Loss of function of an enzyme required to synthesize triglycerides or a nuclear receptor that stimulates differentiation of fat cells make sense. It is less clear why mutations in the gene for nuclear lamins A and C (see Fig. 14-9) or a protease that processes lamin A should cause loss of fat.
Brown Fat Cells
Brown fat cells derive their color from numerous mitochondria, which they use to generate heat in response to cold or (in lean rodents) to excess food intake. Cytochromes make mitochondria brown. Fat is stored in multiple, small droplets (Fig. 28-4F). Brown fat is less abundant than white fat, being concentrated in connective tissue between the scapulae in mammals. Newborn humans have more brown fat than do adults in order to generate heat during the adjustment to a new environment after birth. Hibernating animals use brown fat to raise their temperatures when emerging from hibernation.
Brown fat cells generate heat by short-circuiting the proton gradient that is usually used to generate adenosine triphosphate (ATP) in mitochondria (see Fig. 19-5). Sympathetic nerves acting through β-adrenergic receptors (see Fig. 27-3) and protein kinase A stimulate brown fat cells to express an “uncoupling protein” and to break down lipids to provide fatty acids for oxidation by mitochondria. Uncoupling protein inserts into the inner mitochondrial membrane and dissipates the proton electrochemical gradient across the inner membrane. Energy is lost as heat rather than being used to synthesize ATP. Thermogenesis may be an “energy buffer” that, when defective in animals, can contribute to obesity.
Origin and Development of Blood Cells
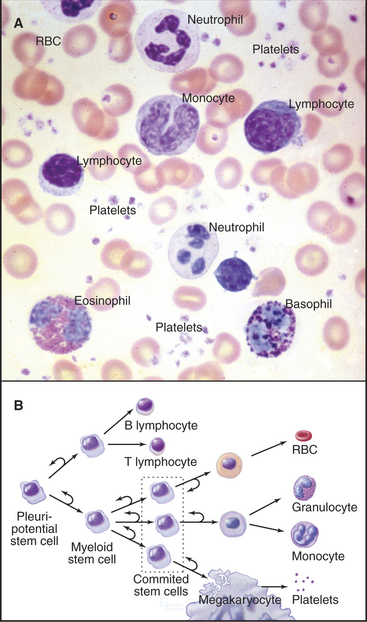
The blood of vertebrates contains a variety of cells, each with a specialized function (Fig. 28-5; Table 28-1). Red blood cells transport oxygen, platelets repair damage to blood vessels, and various types of white blood cells defend against infections. All blood cells derive ultimately from pluripotential stem cells (Fig. 28-5B; also see Box 41-1). After purification, these stem cells can restore the production of all blood cells in mice that have been irradiated to destroy their own blood cell precursors. Destruction of stem cells (e.g., by drugs such as chloramphenicol) leads to aplastic anemia, a condition in which few blood cells are produced, owing to a lack of precursors. These stem cells are also responsible for restoring blood cell production following human bone marrow transplantation.
Table 28-1 BLOOD CELLS (AS SEEN ON A STAINED SMEAR OF BLOOD)
| Type | Concentration | Features |
|---|---|---|
| Platelets | 300,000/μL | Anucleate; 2–3 μm wide; purple granules |
| Erythrocytes | ˜5 × 106/μL | 7-μm; diameter biconcave disks; no nucleus; pink cytoplasm |
| Neutrophils | ˜60% of total WBCs | 10–12 μm wide; multilobed nucleus; many unstained granules; few azurophilic granules |
| Eosinophils | ˜2% of total WBCs | Bilobed nucleus; numerous, large, refractile, pink-stained granules; ˜12 μm wide |
| Basophils | ˜0.5% of total WBCs | Lobed nucleus; large, blue-stained granules; ˜10 μm wide |
| Lymphocytes | ˜30% of total WBCs | Small, round, intensely stained nucleus; some small azurophilic granules; variable amount of clear blue cytoplasm, so they may be classified as either small (˜7–8 μm wide), medium, or large |
| Monocytes | ˜5% of total WBCs | Up to 17 μm wide; large, indented nucleus and gray-blue cytoplasm with a few azurophilic granules |
WBCs, white blood cells.
Proliferation and differentiation of the progeny of pluripotential stem cells produce mature blood cells. At several stages in each line of cells, precursors undergo irreversible differentiation that commits them to a particular lineage. The first branch in the pathway of differentiation separates the precursors of lymphocytes from the precursors of the other blood cells called myeloid stem cells. Next, the myeloid stem cell differentiates into three different committed stem cells. One gives rise to red blood cells; another gives rise to megakaryocytes and platelets. Monocytes and the three types of granulocytes (neutrophils, eosinophils, and basophils) originate from a common committed stem cell in bone marrow and share a number of physiological features. Through differentiation, each also acquires unique functions. Platelets, red cells, granulocytes, and monocytes develop in bone marrow. Lymphocytes develop in bone marrow as well as in lymphoid tissues (thymus, spleen, and lymph nodes).
Minute quantities of specific glycoprotein growth factors control the balance between self-renewal and proliferation at each stage of development, starting with pluripotential stem cells. Feedback mechanisms control production of these growth factors. For example, the oxygen level in the kidney controls the synthesis of erythropoietin, the growth factor for the red blood cell series. (See Fig. 27-9 for the erythropoietin signaling pathway.) A dimeric transcription factor called HIF-1a/HIF-1b regulates the expression of erythropoietin. When oxygen is abundant, HIF-1a is hydroxylated on a proline residue, marking it for ubiquitination and destruction (see Fig. 23-8), turning down the expression of erythropoietin. When kidney cells lack oxygen (owing to low levels of red blood cells, poor blood circulation in the kidney, or high altitude) HIF-1a/HIF-1b accumulates, and erythropoietin is expressed and secreted to stimulate red blood cell production by bone marrow. The reciprocal relationship between oxygen and erythropoietin that is achieved by this feedback mechanism sets red blood cell production at a level required to deliver oxygen to the tissues. Many other cells use the HIF-1a/HIF-1b system to adjust gene expression to local oxygen levels.
Mutations altering the growth control (see Fig. 41-10) of a stem cell can give rise to monoclonal proliferative disorders, such as leukemia. In chronic myelogenous leukemia, a chromosomal rearrangement in a single white blood cell precursor creates a fusion between the genes for BCR (breakpoint cluster region) and ABL (a Src family cytoplasmic tyrosine kinase; see Fig. 25-3 and Box 27-1). The BCR-ABL protein is constitutively active and drives the proliferation of a clone of immature white blood cells that crowd out and inhibit the production of other blood cells, leading to anemia and platelet deficiency. Affected individuals are prone to infection because the immature white blood cells are ineffective phagocytes. Fortunately, a small-molecule inhibitor of the kinase activity of BCR-ABL suppresses this clone in many patients. Uncontrolled proliferation of a clone of red blood cell precursors causes a similar condition, characterized by excess red cells, called polycythe-mia vera.