CARTILAGE FORMATION, GROWTH, & REPAIR
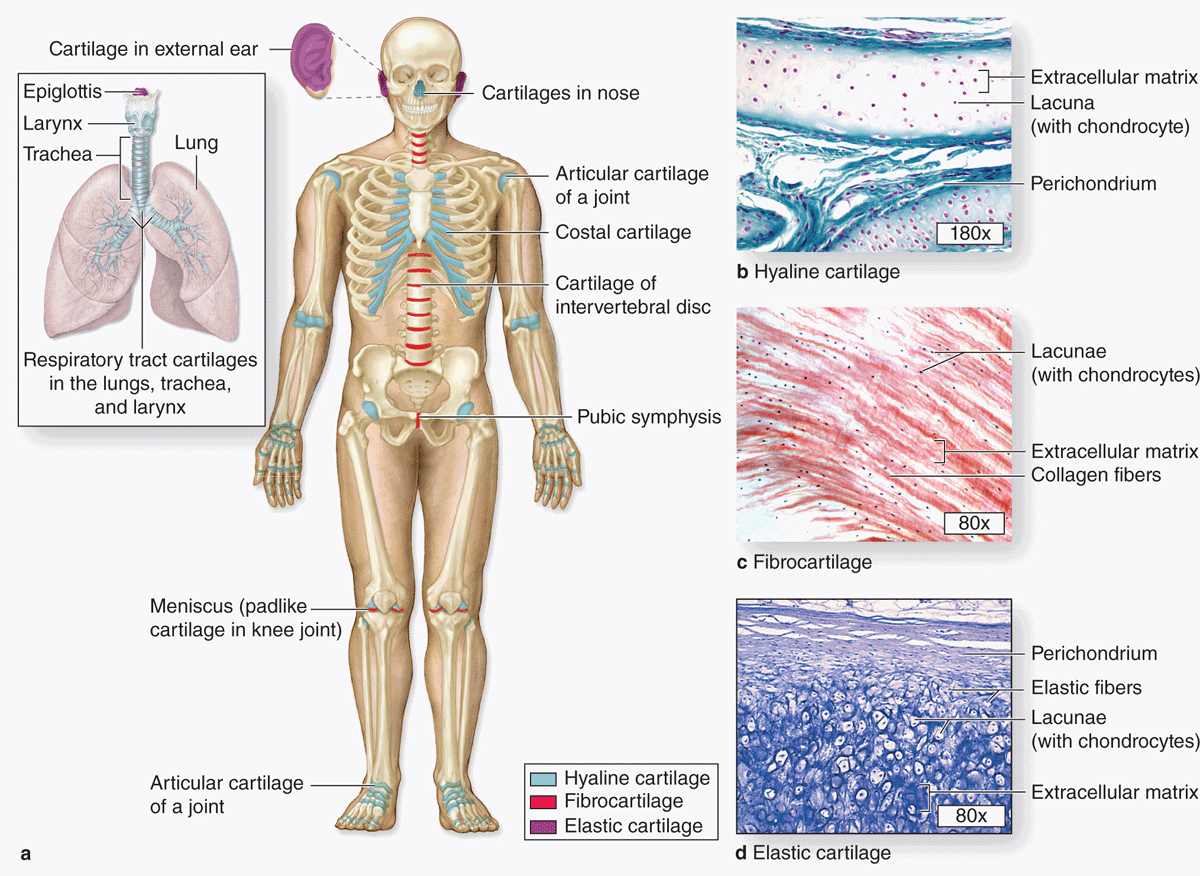
Cartilage is a tough, flexible form of connective tissue, characterized by an extracellular matrix (ECM) with high concentrations of GAGs and proteoglycans, which interact with collagen and elastic fibers. Variations in the composition of these matrix components and cells produce three types of cartilage adapted to local biomechanical needs (Figure 7–1).
FIGURE 7–1 Distribution of cartilage in adults.
The firm consistency of the cartilage ECM allows the tissue to bear mechanical stresses without permanent distortion. In the respiratory tract, ears, and nose, cartilage forms the framework supporting soft tissues. Because of its smooth, lubricated surface and resiliency, cartilage provides shock-absorbing and sliding regions within joints and facilitates bone movements. As described in the next chapter, cartilage also guides development and growth of long bones, both before and after birth.
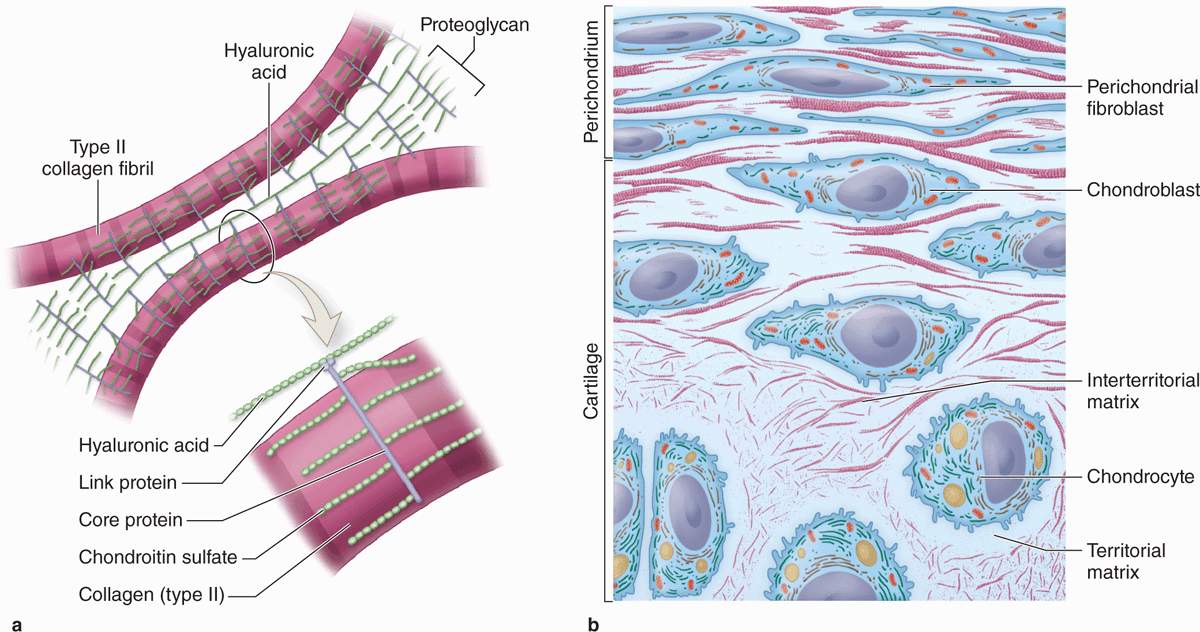
Cartilage consists of cells called chondrocytes (Gr. chondros, cartilage + kytos, cell) embedded in an extensive ECM. Chondrocytes synthesize and maintain ECM components and are located in matrix cavities called lacunae. Collagen, hyaluronic acid, proteoglycans, and various glycoproteins are the principal macromolecules present in all types of cartilage matrix.
The physical properties of cartilage depend on electrostatic bonds between the flexible collagen and elastin fibers and the GAGs on densely packed proteoglycans. Its semi-rigid consistency is attributable to water (solvation water) bound to the negatively charged sulfated GAG chains extending from the proteoglycan core proteins. The high content of bound water allows cartilage to serve as a shock absorber, a role of major functional importance.
Different functional requirements have selected for the three major forms of cartilage, each varying somewhat in matrix composition. In hyaline cartilage, the most common form, type II collagen is the principal collagen type (Figure 7–1). The more pliable and distensible elastic cartilage possesses abundant elastic fibers in addition to collagen type II. Fibrocartilage, present in body regions subjected to pulling forces, is characterized by a matrix containing a dense network of coarse type I collagen fibers.
In all three forms, cartilage is avascular and receives nutrients by diffusion from capillaries in adjacent connective tissue (perichondrium). In some instances, large blood vessels traverse cartilage to supply other tissues, but these vessels release few nutrients to the cartilage. As might be expected of cells in an avascular tissue, chondrocytes exhibit low metabolic activity. Cartilage also lacks lymphatic vessels and nerves.
The perichondrium (Figure 7–2) is a sheath of dense connective tissue that surrounds cartilage in most places, forming an interface between the cartilage and the tissues supported by the cartilage. The perichondrium harbors the cartilage’s vascular supply, as well as nerves and lymphatic vessels. Articular cartilage, which covers the surfaces of bones in movable joints, lacks perichondrium and is sustained by the diffusion of oxygen and nutrients from the synovial fluid.
FIGURE 7–2 The structure of cartilage matrix and cells.
HYALINE CARTILAGE
Hyaline (Gr. hyalos, glassy) cartilage (Figure 7–1), the most common of the three forms, is homogeneous and semitransparent in the fresh state. In adults hyaline cartilage is located in the articular surfaces of movable joints, in the walls of larger respiratory passages (nose, larynx, trachea, bronchi), in the ventral ends of ribs, where they articulate with the sternum, and in the epiphyseal plates of long bones, where it makes possible longitudinal bone growth. In the embryo, hyaline cartilage forms the temporary skeleton that is gradually replaced by bone.
Matrix
The dry weight of hyaline cartilage is 40% collagen embedded in a firm, hydrated gel of proteoglycans and structural glycoproteins. In routine histology preparations, the proteoglycans cause the matrix to be generally basophilic and the thin collagen fibrils are barely discernible. Most of the collagen in hyaline cartilage is type II collagen, although small amounts of several minor types are also present.
Aggrecan (250 kD), with approximately 150 GAG side chains of chondroitin sulfate and keratan sulfate, is the most abundant proteoglycan of hyaline cartilage. Hundreds of these proteoglycans are bound noncovalently by link proteins to long polymers of hyaluronic acid, as shown schematically in Figure 7–2a and discussed in Chapter 5. These proteoglycan complexes bind further to the surface of type II collagen fibrils (Figure 7–2a). Water bound to GAGs in the proteoglycans constitutes up 60%-80% of the weight of fresh hyaline cartilage.
Another important component of cartilage matrix is the structural multiadhesive glycoprotein chondronectin. Like fibronectin in other connective tissues, chondronectin binds specifically to GAGs, collagen type II, and integrins, mediating the adherence of chondrocytes to the ECM.
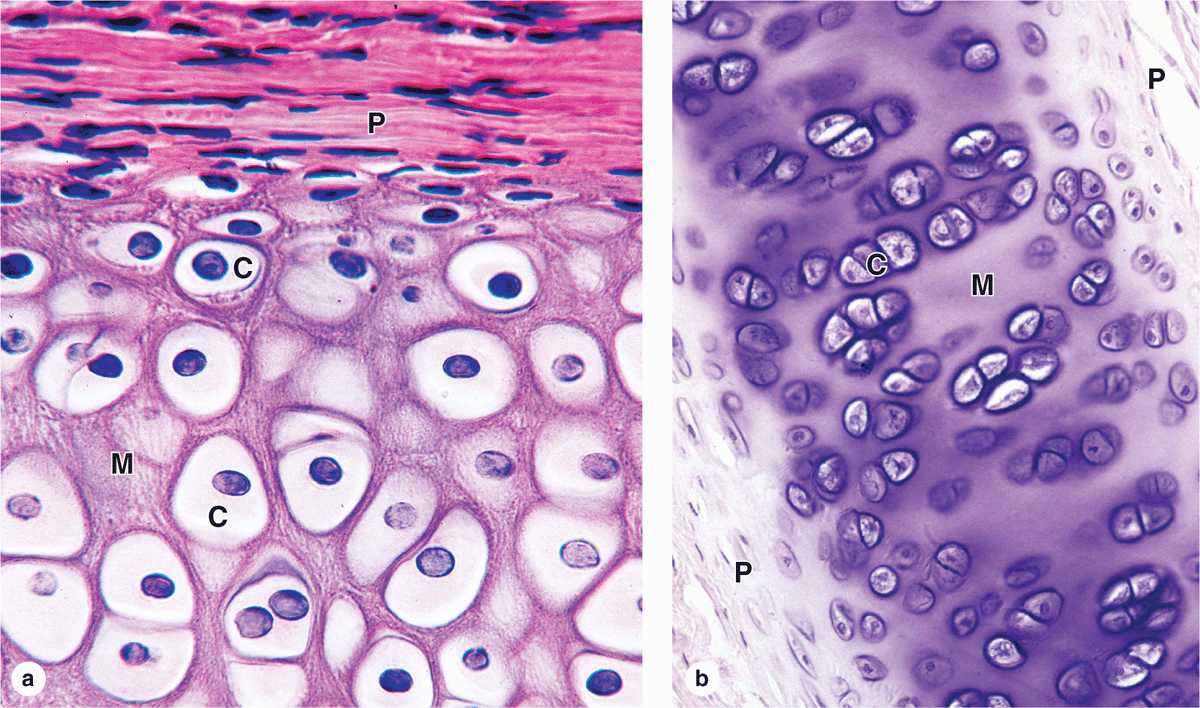
Staining variations within the matrix reflect local differences in its molecular composition. Immediately surrounding each chondrocyte, the ECM is relatively richer in GAGs, often causing these areas of territorial matrix to stain differently from the intervening areas of interterritorial matrix (Figures 7–2b and 7–3.
FIGURE 7–3 Hyaline cartilage.
 MEDICAL APPLICATION
MEDICAL APPLICATION MEDICAL APPLICATION
MEDICAL APPLICATION