Chapter 4 CARDIOVASCULAR DISEASES
Introduction
 More than 50% of all deaths in the United States and Western Europe are caused by cardiovascular diseases.
More than 50% of all deaths in the United States and Western Europe are caused by cardiovascular diseases.
 Atherosclerosis is the most common chronic multifactorial degenerative disease and is especially prevalent among the elderly.
Atherosclerosis is the most common chronic multifactorial degenerative disease and is especially prevalent among the elderly.
 Hypertension affects millions of Americans and is one of the most prevalent chronic diseases.
Hypertension affects millions of Americans and is one of the most prevalent chronic diseases.
 Congenital heart diseases are found in 5 per 1000 newborns.
Congenital heart diseases are found in 5 per 1000 newborns.
 Many cardiovascular diseases, such as atherosclerosis, are preventable or treatable if recognized early (e.g., arterial hypertension). It is thus encouraging to note that the incidence of cardiovascular disease has decreased in the United States over the last 40 years. Public health initiatives to reduce smoking, combat hyperlipidemia, and motivate people to exercise and follow a healthier life style have had noticeable effects on the incidence of cardiovascular diseases and their major complications. Major advances have been made also in the early diagnosis of cardiovascular disease, treatment based on interventional cardiology, and heart transplantation.
Many cardiovascular diseases, such as atherosclerosis, are preventable or treatable if recognized early (e.g., arterial hypertension). It is thus encouraging to note that the incidence of cardiovascular disease has decreased in the United States over the last 40 years. Public health initiatives to reduce smoking, combat hyperlipidemia, and motivate people to exercise and follow a healthier life style have had noticeable effects on the incidence of cardiovascular diseases and their major complications. Major advances have been made also in the early diagnosis of cardiovascular disease, treatment based on interventional cardiology, and heart transplantation.
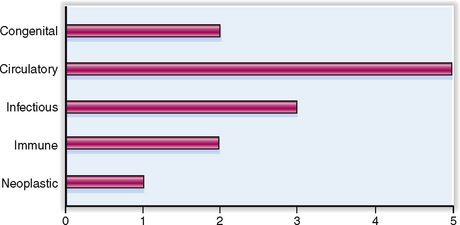
Cardiovascular diseases have many causes, but most of them are multifactorial. The relative clinical significance of various cardiovascular diseases is presented graphically in Figure 4-1.
Anatomy and Physiology
Actin Contractile protein that with myosin forms the filaments in sarcomeres.
Angiotensin Vasoactive substance formed from angiotensinogen through the action of renin. It plays an important role in the pathogenesis of hypertension.
Aorta Largest artery in the body, conveying arterial blood from the heart to the periphery.
Arteriole Small muscular vessel serving as precapillary sphincter at the interface between the muscular arteries and capillaries.
Artery Thick-walled muscular or elastic blood vessel conveying arterial blood from the heart to the arterioles.
Atrial natriuretic peptide Family of vasoactive polypeptide hormones produced by the heart and vascular cells; important for the regulation of heart function and blood pressure.
Atrium Chamber of the heart connected to systemic or pulmonary veins on one side and separated by a valve from the ventricle on the other. The heart has two atria: the right and the left atrium.
Conduction system of the heart System of specialized cells capable of generating and conducting the electric currents essential for the automatic rhythmicity of the heart. It includes the sinoatrial node, atrioventricular node, the bundle of His and its main branches, and Purkinje fibers.
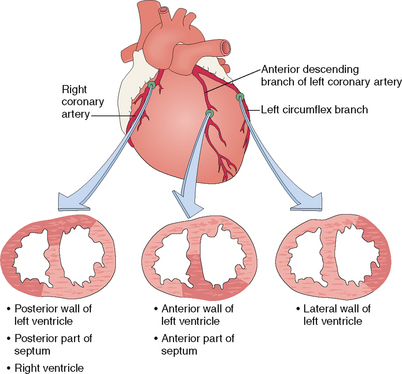
Coronary arteries Arteries providing blood to the heart. Two major arteries originate in the aorta from the coronary sinuses. The left coronary artery has two main branches: the left anterior descending (LAD), providing the blood to the anterior side of the left ventricle and the anterior part of the ventricular septum; and the left circumflex (LCX), which provides blood to the lateral side of the left ventricle. The right coronary artery (RCA) usually gives rise to the posterior descending artery (PDA). The PDA originates from the RCA in 90% of the population designated as having right coronary dominance. In 10% of cases left coronary dominance occurs, and the PDA originates from the LCX.
Endocardium Inner layer of the heart, forming the surface of the cardiac chambers. It is continuous with the endothelium of the great vessels.
Frank-Starling law The power of ventricular contraction increases proportionately to the initial lengthening of its muscle fibers by end-diastolic filling.
Myocardium Thickest layer of the atria and ventricles, composed of contractile cardiac myocytes.
Myoglobin Oxygen-binding heme protein found in cardiac and skeletal muscle cells. It may spill out into the blood from necrotic muscle fibers and is thus a marker of myocardial cell necrosis occurring after myocardial infarction.
Myosin Contractile protein forming the microfilaments in many human cells and most prominently the myofibrils in cardiac myocytes.
Pericardium Outer layer of the heart, composed of a mesothelial layer and underlying connective tissue. It covers the subepicardial fat tissue, which contains the branches of the coronary arteries and veins.
Renin Polypeptide hormone synthesized by juxtaglomerular (JG) cells in the kidney. It acts on angiotensinogen and is thus important for the aldosterone-mediated retention of sodium and water in the kidneys. It is synthesized in response to hypoperfusion of kidneys and is the primary mediator of renal hypertension.
Sarcomere Functional contractile unit of each cardiac myocyte. It consists of thick filaments (myosin) and thin filaments (actin) and the regulatory proteins, troponins and tropomyosin.
Sarcoplasmic reticulum Smooth endoplasmic reticulum in cardiac myocytes, composed of interconnected vesicles that serve as a calcium reservoir of these cells.
Septum Part of the heart separating the left atrium from the right atrium, and the left ventricle from the right ventricle. It consists of fibrous tissue (fibrous septum) and muscle (muscular septum).
Troponin I and T Sarcomeric proteins regulating the contraction of myofibrils. In myocardial infarction these proteins are released into the blood. The elevation of troponin I or T in blood is evidence of myocardial necrosis.
Valve Mobile part of the cardiac orifice that separates atria from ventricles or the ventricles from the great vessels. Valves consist of a fibroelastic core covered with the endocardium. The mitral and tricuspid valves are attached to the papillary muscles with chordae tendineae. The aortic and pulmonary valves are semilunar and not attached to the muscles. At their base the valves are anchored to the annulus fibrosus, forming the connective tissue skeleton of the heart.
Ventricle Cardiac chamber that has a thick muscular wall accounting for most of the contractile power of the heart. The heart has two ventricles, the right and the left ventricle, separated one from each other by a predominantly muscular septum.
Signs and Symptoms
Arrhythmia Collective term used to describe several abnormalities of heartbeat, it includes, among others, tachycardia, bradycardia, premature ventricular beat, atrial or ventricular fibrillation, flutter, conduction defects, and heart block.
Bradycardia Slow heartbeat, usually under 50 beats per minute.
Clubbing of fingers Condition in which the amount of subcutaneous soft tissue of the terminal phalanges is increased, with curving of the nails, giving the fingers a drumstick appearance. It is commonly seen in cyanotic congenital heart diseases, such as tetralogy of Fallot. It is also encountered in chronic lung diseases with long-standing ventilatory problems.
Congestive hepatomegaly Enlargement of the liver due to chronic passive accumulation of venous blood in the liver owing to right heart failure.
C-reactive polypeptide, or protein (CRP) Acute-phase reactant secreted by the liver in response to cytokines released from inflammatory cells. Its concentration is elevated in blood in various inflammatory conditions, including the inflammation that occurs inside atherosclerotic blood vessels. As such it is an independent predictor of the risk for complications of atherosclerosis.
Creatine kinase (CK) Enzyme released from damaged or necrotic cardiac and skeletal muscle. The cardiac isoenzyme (CK-MB) is elevated in the blood after myocardial infarction.
Cyanosis Bluish or purplish tinge to the skin and mucosae that becomes visible once the unoxygenated hemoglobin concentration exceeds 5 g/100 mL of blood, or the overall oxygen saturation is less than 85%. It may be classified as central or peripheral.
Dyspnea Shortness of breath that may be acute or chronic, episodic or continuous, exertional or resting. Usually it is a symptom of heart or lung disease. Exertional dyspnea or paroxysmal nocturnal dyspnea is usually a sign of heart disease.
Edema Accumulation of fluid in tissues and body cavities. Cardiogenic edema involves either lungs and pleural cavity (in left heart failure) or peripheral tissues of the lower extremities and abdominal cavity (in right heart failure).
Hypertension Elevated blood pressure in the arteries. It may be primary or idiopathic (90%), if the cause of hypertension is unknown, or secondary if the cause of hypertension is known. The most common causes of secondary hypertension are renal, endocrine, or vascular diseases.
Intermittent claudication Sudden pain in the lower extremities, precipitated by walking; it is so severe that it will cause limping or might force the affected person to stop walking. It is typically a consequence of atherosclerotic narrowing of major leg arteries.
Ischemia Localized circulatory disturbance resulting in the interruption of blood flow into an anatomic area. Most often caused by thrombotic or atheromatous occlusion of the arterial lumen.
Orthopnea Shortness of breath that occurs when the patient lies down. It is usually a sign of lung disease because the vital capacity is normally reduced in supine position.
Paroxysmal nocturnal dyspnea Form of dyspnea that occurs in patients with heart failure several hours after lying down. It is caused by increased venous return and the failure of the heart to propel the blood, leading to pulmonary congestion and edema and shortness of breath that wakes the patient.
Pulmonary edema Accumulation of fluid in the alveoli and interstitial spaces of the lung. It may be a consequence of transudation of serum in left ventricular failure, during early stages of ARDS, or as an inflammatory exudate in early stages of pneumonia.
Pulmonary hypertension Increased pressure inside the pulmonary artery and its branches. It may be a consequence of primary lung disease or chronic pulmonary congestion due to left heart failure or mitral valve disease. Pulmonary hypertension poses a strain on the right ventricle, causing its hypertrophy (“cor pulmonale”).
Syncope Loss of consciousness due to hypoperfusion of the brain by blood.
Tachycardia Rapid heartbeat, in adults usually over 90 heartbeats per minute.
Cardiovascular Diseases and Pathologic Lesions
Amyloidosis of the heart Deposition of fibrillar extracellular material that binds Congo red and is β-pleated when examined by electron diffraction analysis. It may be part of generalized amyloidosis or may present as an isolated cardiac amyloidosis. Deposits of amyloid in the cardiac interstitium cause restrictive cardiomyopathy.
Anastomosis Connection between two vessels or two hollow organs. Vascular anastomoses may be between two arteries or two veins or they may be arteriovenous (A-V).
Aneurysm of aorta or arteries Localized dilatation of an artery, caused most often by atherosclerosis, but also by syphilis or various forms of arteritis. Berry aneurysms of the circle of Willis are congenital.
Aneurysm of the ventricle Dilatation of the ventricular lumen, most often as a late complication of myocardial infarction. The wall of the aneurysm is composed of fibrous scars and does not contract with the surrounding myocardium. Thrombi form within large ventricular aneurysms.
Angina pectoris Chest pain caused by cardiac ischemia related to stenosis, partial occlusion, or spasm of the coronary arteries. Three main forms are recognized: (1) stable or classical angina; (2) unstable or crescendo angina or preinfarctional angina; and (3) Prinzmetal’s angina, which is characterized by pain at rest, caused by functional narrowing of coronary arteries due to spasm.
Atheroma Hallmark of atherosclerosis, presenting as a bulging of the wall of the aorta or a circumferential narrowing of smaller elastic arteries. It consists of a central core composed of lipids, cell debris, and foam cells, and a fibrous cap covering it on the luminal side. Complicated atheromas may be calcified, or ruptured and filled with coagulated blood.
Atherosclerosis Common multifactorial disease of aorta and major elastic arteries, causing numerous diseases, such as coronary artery disease and cerebrovascular accidents (strokes).
Atrial fibrillation Disturbance of normal atrial contraction, which is replaced by irregular twitching, best recognized by electrocardiogram (ECG). May predispose patient to formation of atrial mural thrombi.
Atrioventricular (AV) block Disturbance of cardiac conduction characterized by an inability to transmit electric signals from the atrium to the ventricles.
Calcific aortic stenosis Narrowing of the aortic orifice due to the calcification of the valves. It is most often caused by wear and tear of old age, but can be a complication of congenital bivalvular aortic orifice and rheumatic endocarditis.
Carcinoid heart disease Cardiac disease caused by serotonin and other vasoactive substances released from gastrointestinal carcinoids that have metastasized to the liver. Cardiac changes include tricuspid and pulmonary valve stenosis and insufficiency and fibrosis of the atrial and ventricular lumen resulting from subendocardial fibrosis induced by vasoactive substances.
Cardiac tamponade Compression of the heart by fluid, usually blood, that has entered into the pericardial sac. The pressure exerted by the fluid prevents the diastolic filling of the cardiac chambers and is usually fatal.
Cardiogenic shock Systemic circulatory collapse caused by cardiac pump failure. It is characterized by hypotension and hypoperfusion of vital organs, causing ischemia of the brain, heart, kidneys, and other organs. Most often it is a complication of myocardial infarction.
Cardiomyopathy Term used to describe primary myocardial diseases unrelated to coronary atherosclerosis, hypertension, or infectious or rheumatic carditis. Cardiomyopathy may occur in three forms: (1) dilated cardiomyopathy, (2) hypertrophic cardiomyopathy, and (3) restrictive cardiomyopathy.
Congestive heart failure Clinical condition characterized by stagnation of blood retrograde to the failing heart. Left heart failure causes chronic passive congestion and edema of the lungs, whereas right heart failure results in congestive hepatomegaly, pitting edema of the lower extremities, ascites, and splenomegaly.
Constrictive pericarditis Form of chronic pericarditis or pericardial fibrosis encasing the heart and preventing its expansion during diastole. The cause of constrictive pericarditis is most often unknown, and it is assumed to be a complication of a healed viral pericarditis. Radiation, tuberculosis, or tumors can cause the same changes.
Cor pulmonale Clinical condition characterized by dilatation and strain of the right ventricle and possible right ventricular failure. Most often it is caused by left ventricular failure, but also by any other condition causing pulmonary hypertension.
Dilatation of the heart Widening of the cardiac chambers, usually due to the stretching of cardiac myocytes. Dilatation of the ventricles is the first adaptation to an increased demand for cardiac output, and during this response the dilatation is beneficial and reversible.
Endocarditis, infectious Inflammation caused by a bacterial infection of the valves or mural endocardium of the heart chambers. Bacteria destroy the valves, causing valvular defects and disturbing blood flow; emboli may occur and cause systemic symptoms.
Floppy valves Congenital or acquired myxomatous degeneration of the valves accompanied by accumulation of sulfated glycosaminoglycans and a loss of collagen and elastic fibers. Mitral valve disease is found in 1% to 2% of adults; other valves are involved less often.
Hemopericardium Accumulation of blood in the pericardial cavity. It may be caused by bleeding from the cardiac chambers or the root of the aorta.
Hypertensive heart disease Complex heart disease caused by elevated arterial pressure. It is characterized by left ventricular hypertrophy and relative ischemia of the myocardium, which cannot get enough blood for its needs. Hypertension also predisposes to coronary atherosclerosis, which will contribute to myocardial ischemia.
Ischemic heart disease (IHD; also known as coronary heart disease) Group of circulatory diseases caused by inadequate oxygen due to coronary insufficiency. Most often it is caused by atherosclerotic narrowing or occlusion of coronary arteries. The most important clinical forms of IHD are (1) angina pectoris, (2) myocardial infarct, (3) congestive heart disease, and (4) sudden cardiac death.
Left ventricular failure Pump failure of the left ventricle manifesting with hypoperfusion of organs with arterial blood (forward failure) and stagnation of the blood in the lungs. It manifests with pulmonary congestion and edema.
Left ventricular hypertrophy Thickening of the left ventricular myocardium. Most often it is caused by arterial hypertension, but it may also be caused by stenosis of the aortic orifice or insufficiency of the mitral valve. It is also found in cardiomyopathies.
Libman-Sacks endocarditis Sterile endocarditis typically found in systemic lupus erythematosus. Most often it affects the mitral valve.
Mitral valve incompetence (also known as mitral insufficiency or mitral regurgitation) Functional disturbance resulting from the inability of the mitral valves to close completely during systole.
Mitral valve prolapse Synonym for floppy mitral valve (see Floppy valves)
Mitral valve stenosis Functional disturbance resulting from the narrowing of the mitral orifice and valves, which cannot open completely during diastole. Most often (99%) it is a consequence of rheumatic endocarditis.
Myocardial infarct, subendocardial Ischemic necrosis of the subendocardial zone of the myocardium, most often circumferential. Typically caused by hypoperfusion without complete anatomic occlusion of a coronary artery. It is most often found in shock or as a complication of myocardial infarction. In typical cases it presents without Q wave changes in the ECG (“non-Q wave infarction”), and is not accompanied by pericarditis.
Myocardial infarct, transmural Localized area of ischemic necrosis of the myocardium caused by occlusion of a coronary artery. Most often it is a complication of thrombosis developing at the site of ruptured atheroma. Thomboemboli from the left ventricle are a less common cause.
Myocarditis Inflammation of the myocardium accompanied by necrosis of cardiac myocytes. Etiologically classified as (1) infectious or (2) noninfectious.
Myxoma Benign cardiac tumor most often found in the left atrium (75%), less often in the right atrium (24%), and very seldom in other cardiac chambers. Tumor protrudes into the lumen of the atria and may act as a “ball-valve.” As such, it may temporarily occlude the mitral or tricuspid orifice and interrupt the blood flow. Also, parts of the tumor may detach and thus give rise to emboli.
Myxomatous degeneration of cardiac valves Deformation of cardiac valves accompanied by accumulation of sulfate glycosaminoglycans and a loss of normal collagen and elastic fibers forming the central core of the valves. In most instances the cause is unknown. It may occur in inborn errors of metabolism such as the Marfan’s syndrome and Ehlers-Danlos syndrome or various mucopolysaccharidoses. The mitral valve is most often involved, but all valves can be affected.
Pericarditis Inflammation of epicardium and pericardium (i.e., the visceral and parietal layer of the pericardial sac). It can manifest in several pathologic forms: serous, fibrinous, fibrinohemorrhagic, purulent, fibrous, or calcific.
Rheumatic heart disease Immune-mediated disease initiated by antigens on beta-hemolytic streptococci, usually after a streptococcal pharyngitis (“strep throat”). Clinically it is diagnosed by documenting the rheumatic fever (using the Jones criteria), establishing the link between the heart disease and the streptococcal infection (ASO titers high), and proving that the heart is damaged. Rheumatic carditis most often affects the cardiac valves of the left side of the heart.
Right ventricular hypertrophy Thickening of the wall of the right ventricle, typically caused by pulmonary hypertension. The most common cause of right ventricular hypertrophy is left ventricular hypertrophy.
Rupture of the ventricle Perforation of the wall of the left ventricle, usually caused by ischemic necrosis (transmutable infarct). Blood penetrates through the ventricular wall, filling the pericardial cavity and causing death due to pericardial tamponade.
Shunt A passage that allows abnormal blood flow between two cardiac chambers on the opposite sides of the septum dividing the left from the right heart. Blood flows through the shunt from the higher pressurized chamber to the chamber that is under less pressure.
Stenosis Narrowing of the lumen of a blood vessel or a cardiac orifice.
Tamponade (more correctly “cardiac tamponade”) Compression of the heart by the fluid accumulating in the pericardial sac. The most common cause of cardiac tamponade is hemopericardium.
Tricuspid valve insufficiency Functional disturbance resulting from incomplete closure of the tricuspid valves. May be congenital or acquired. Acquired tricuspid valve insufficiency can be a consequence of endocarditis or carcinoid heart disease.
Truncus arteriosus Congenital heart disease related to incomplete separation of the aorta from the fetal pulmonary artery. In this disease the aorta and the pulmonary artery form a single large blood vessel originating from the heart.
Valvular calcification Dystrophic calcification affecting valves previously damaged by endocarditis, or resulting from the wear and tear of old age. Congenitally abnormal or lax valves are especially prone to calcification.
Valvular insufficiency Circulatory disturbance caused by incomplete closure of a cardiac valve, resulting in regurgitation of blood from one chamber of the heart or a great vessel into another one of the heart chambers.
Valvular stenosis Circulatory disturbance caused by narrowing of the orifice, usually caused by incomplete opening of the valves during systole or diastole.
Normal Structure and Function
ANATOMY AND HISTOLOGY
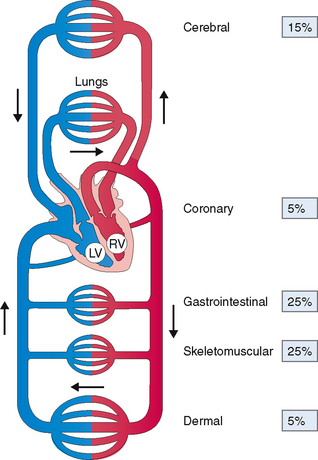
The cardiovascular system comprises the heart and blood vessels, which are classified as arteries, arterioles, capillaries, venules, and veins. The right heart pumps the blood into the lungs, where it is oxygenated and returned to the left heart for distribution through several parallel arterial pathways into the peripheral tissues (Fig. 4-2). This chapter focuses mostly on the heart, and the blood vessels are discussed only as they contribute to heart diseases and their peripheral manifestations.
The heart is located in the mediastinum of the thoracic cavity.
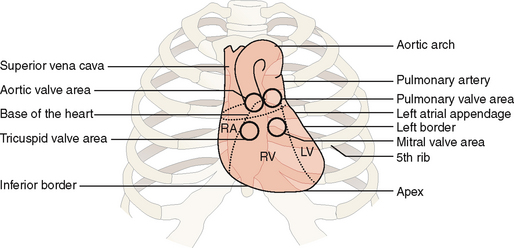
The heart is located inside the pericardial sac, which is itself located in the lower (supradiaphragmatic) part of the mediastinum. The heart occupies the central portion of the mediastinum, extending slightly to the right from the midline and projecting with its apex toward the left lateral side (Fig. 4-3). The great vessels (i.e., the superior and inferior vena cava, the aorta, the pulmonary artery, and the pulmonary veins) are attached to the heart at its “base,” which is located cranially and to the right of the apex. The contours of the heart are clearly visible in anteroposterior (AP) radiographic films.
In adults the anterior mediastinum contains the remnants of the thymus, which does not affect the function of the heart unless enlarged due to thymoma or lymphoma. The posterior side of the heart is in anatomic contact with the esophagus and the thoracic aorta. Transesophageal echocardiography may be used to assess the left atrium lying on the esophagus, but it may also provide data on the entire heart.
The heart has four chambers and can be functionally divided into a right and a left part.
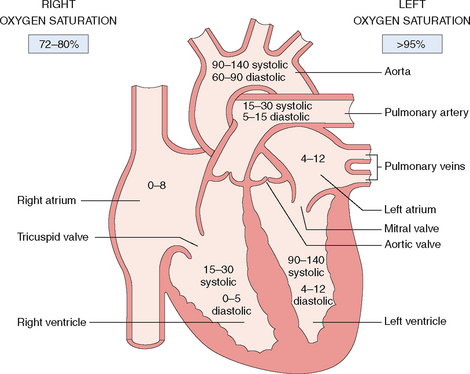
The heart has two atria and two ventricles (Fig. 4-4). The right atrium and ventricle contain unoxygenated (“venous”) blood, which has an oxygen saturation between 72% and 80%. The contracting right heart pumps the blood into the lungs, where is it oxygenated. The left atrium and ventricle contain oxygenated (“arterial”) blood with an oxygen saturation of 95% or more. The left ventricle propels the oxygenated blood into the periphery through the aorta and the arteries.
The entire heart, except the endocardium, receives oxygenated blood through two coronary arteries.
Most human hearts have two coronary arteries, which originate from the coronary sinuses in the initial part of the thoracic aorta (Fig. 4-5).
The coronary arteries are located subepicardially on the external surface of the heart. The major veins follow the arteries. Because of their location these major vessels are not compressed by the contracting myocardium during the systole—a period of the cardiac cycle when the coronaries actually dilate to accommodate the influx of oxygenated blood. Coronary arteries have β2-adrenergic receptors, which react to stimuli from sympathetic nerves, thereby causing coronary vasodilatation. Parasympathetic stimulation also causes mild coronary vasodilatation.
The heart is composed of three layers: endocardium, myocardium, and pericardium.
 The conducting cells form the SA and AV node, the intra-arterial atrial internodal tracts, the AV node, the bundle of His, and the Purkinje system.
The conducting cells form the SA and AV node, the intra-arterial atrial internodal tracts, the AV node, the bundle of His, and the Purkinje system.
 The contractile myocytes form the bulk of the heart wall. Cardiac myocytes are striated muscle cells with centrally placed nuclei and well-developed cytoplasm. These branching cells are interconnected one with another through gap junctions called intercalated discs allowing easy passage of electric current through the myocardium.
The contractile myocytes form the bulk of the heart wall. Cardiac myocytes are striated muscle cells with centrally placed nuclei and well-developed cytoplasm. These branching cells are interconnected one with another through gap junctions called intercalated discs allowing easy passage of electric current through the myocardium.
Contractile function of cardiac myocytes depends on the proper functioning of sarcomeres and supporting structures in the cytoplasm.
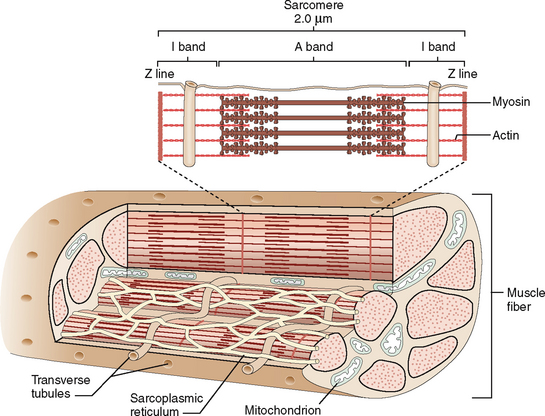
Contractile units in the cytoplasm of cardiac myocytes are called sarcomeres (Fig. 4-6). They are composed of thick myosin filaments interdigitating with thinner actin filaments and a number of contraction-regulating proteins, such as tropomyosin and troponins (C, I, and T). During contraction the myosin filaments slide along one another, whereas during relaxation they slide in the opposite direction.
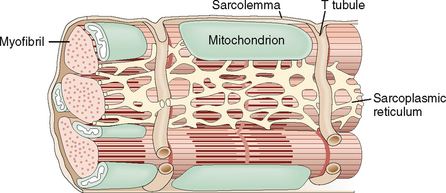
The cell membrane of myocytes, also known as sarcolemma, invaginates into the cytoplasm, forming the so-called transverse tubular system (T tubules). The T system is an important regulator for the flux of calcium ions from outside into the cells and from one cellular compartment to another (Fig. 4-7). The T tubules are closely linked to the sarcoplasmic reticulum, a system of cisterns that serve as the main intracellular store of calcium. The sarcoplasmic reticulum and the T tubules form an integral system essential for the rapid transmission of electrical stimuli and the contraction–relaxation of myocytes.
PHYSIOLOGY
Cardiac contractions depend on the interaction of the external innervation and the endogenous conduction system of the heart.
The heart receives parasympathetic innervation from the vagus and sympathetic innervation from sympathetic ganglia. The cholinergic parasympathetic stimuli, which are under normal circumstances predominant, stimulate the heart through the AV and SA node. Vagal stimulation has a negative chronotropic and inotropic effect; that is, it slows down the heartbeat and reduces the strength of the cardiac contraction. Sympathetic stimuli transmitted mostly through β1-adrenergic receptors have a positive chronotropic and inotropic effect. Sensory nerves, important for the cardiac reflexes, exit the heart through the vagus nerve.
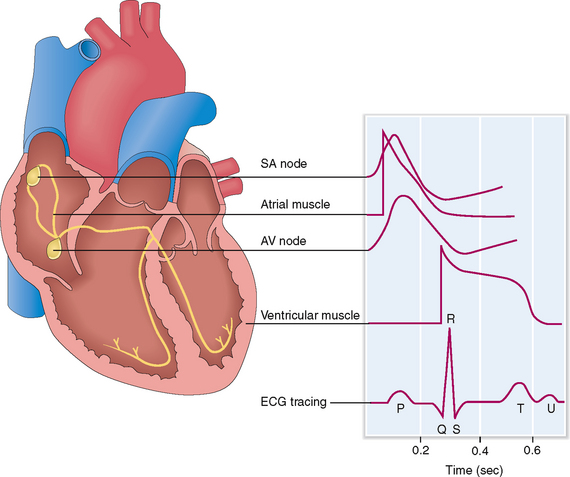
The automatic contractility of the heart is regulated through the SA and AV node and the conduction system of the myocardium. The SA node is the source of initial impulses and accounts for the normal “sinus rhythm.” The depolarization wave is transmitted from the SA node to the internodal and interatrial fibers, which causes depolarization of the right and left atrium. The electric impulses cannot freely pass into the ventricle because the annulus fibrosus blocks their propagation. The only way that the impulses can get into the ventricle is by reaching first the AV node located on the atrial side of the annulus fibrosus. From the SA node the electric impulses enter the bundle of His, which penetrates the annulus fibrosus and allows the stimuli to pass from the atrium to the ventricle. Then they reach the Purkinje fibers and are transmitted to cardiac myocytes, causing their depolarization. These electrical events can be traced externally and if superimposed on one another can be recorded as an electrocardiographic tracing (Fig. 4-8).
Cardiac myocyte contraction begins with the depolarization of the sarcolemma.
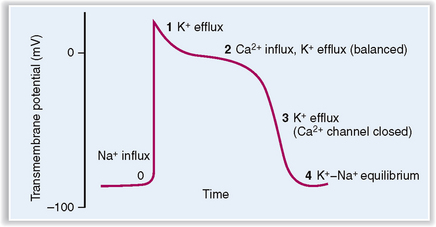
The cardiac myocyte’s action potential, or the change in the membrane potential that occurs after the cell has been adequately stimulated, can be divided into five phases (Fig. 4-9):
 Phase 0 (fast upstroke) characterized by an opening of fast Na+ channels and an influx of sodium. Upstroke ends when the Na+ channels are inactivated.
Phase 0 (fast upstroke) characterized by an opening of fast Na+ channels and an influx of sodium. Upstroke ends when the Na+ channels are inactivated.
 Phase 1 (partial repolarization) resulting from inactivation of Na+ channels and rapid opening and closure of K+ channels. Efflux of K+ accounts for partial repolarization.
Phase 1 (partial repolarization) resulting from inactivation of Na+ channels and rapid opening and closure of K+ channels. Efflux of K+ accounts for partial repolarization.
 Phase 2 (plateau) resulting from the opening of the voltage-sensitive Ca2+ channels, which allows the influx of Ca2+ counterbalanced by a slow efflux of K+.
Phase 2 (plateau) resulting from the opening of the voltage-sensitive Ca2+ channels, which allows the influx of Ca2+ counterbalanced by a slow efflux of K+.
 Phase 3 (repolarization) due to closure of Ca2+ channels and an efflux of K+, leading to repolarization of the cell membrane. The excess of Na+ inside the cell and a net loss of K+ are corrected by active pumping of Na+ and K+ across the cell membrane by Na+/K+ ATPase.
Phase 3 (repolarization) due to closure of Ca2+ channels and an efflux of K+, leading to repolarization of the cell membrane. The excess of Na+ inside the cell and a net loss of K+ are corrected by active pumping of Na+ and K+ across the cell membrane by Na+/K+ ATPase.
 Phase 4 (forward current) characterized by mild gradual depolarization of cell membrane due to Na+ influx accompanied by mild K+ efflux. During this phase the cell rests, and potential rises slowly to reach the initial threshold for the next depolarization.
Phase 4 (forward current) characterized by mild gradual depolarization of cell membrane due to Na+ influx accompanied by mild K+ efflux. During this phase the cell rests, and potential rises slowly to reach the initial threshold for the next depolarization.
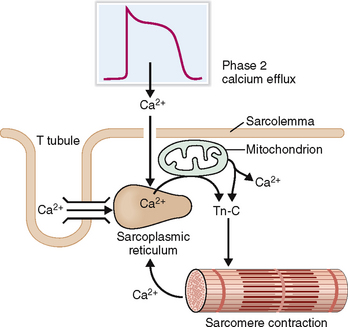
Contraction of sarcomeres requires a marked increase of cystosolic calcium concentration so that it could start binding to troponin C.
During phase 2 of the myocyte action potential Ca2+ enters into the cytoplasm through the calcium channels and is transferred into the sarcoplasmic reticulum (Fig. 4-10). The entry of extracellular Ca2+ acts as a trigger for discharge of large amounts of Ca2+ stored inside the cisterns of the sarcoplasmic reticulum and to a lesser degree in mitochondria. Once the concentration of cytosolic calcium has risen 10-fold Ca2+ binds to troponin C, which inactivates troponin I, allowing it to induce conformational changes in tropomyosin, the principal regulator of actin–myosin interaction. Tropomyosin deblocks actin, allowing it to interact with myosin. This interaction is a multistep process that ultimately leads to contraction of the sarcomeres.
Contraction of the myocytes results in systole and the expulsion of the blood from the ventricles, whereas during diastole the ventricles dilate and fill with blood.
The cardiac cycle has two phases: systole, during which the myocardium of the ventricles contracts, and diastole, during which the ventricles dilate. The force of cardiac contraction is influenced by several factors, the most important of which are the preload, contractility of the myocytes, and the afterload (Fig. 4-11).
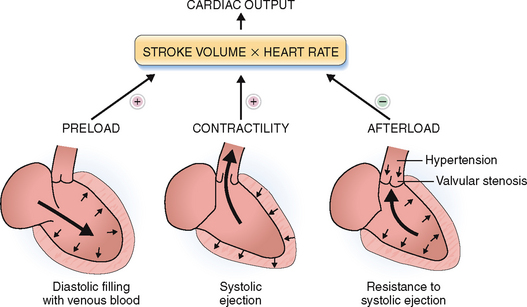
Figure 4-11 Cardiac output depends on the preload, the afterload, and the contractility of the heart.
(Modified from Price AS, Wilson LM: Pathophysiology. Clinical Concepts of Disease Processes, 6th ed. Mosby, St. Louis, 2003.)
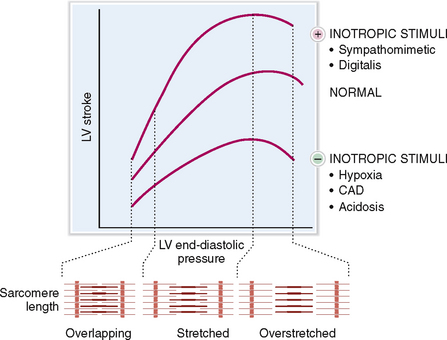
Preload refers to the end-diastolic volume (i.e., the amount of blood in the ventricles just before the contraction). The contraction of the ventricles increases in proportion to the increased preload (i.e., the venous return to the heart). This is in keeping with the Frank-Starling law of the heart, which states that “volume of blood ejected by the ventricle depends on the volume present in the ventricle at the end of diastole.” Many years after Frank and Starling defined their law, it was shown that the increased end-diastolic pressure leads to lengthening of the sarcomeres, which thereupon contract more forcefully (Fig. 4-12). In other words the energy of contraction is proportional to the initial length of the cardiac muscles. The Frank-Starling law works only within limits: if the optimal lengthening of sarcomere is exceeded, the contractions become less powerful.
The action of the heart occurs in repetitive cycles, which include coordination of myocardial contractility and opening and closing of valves.
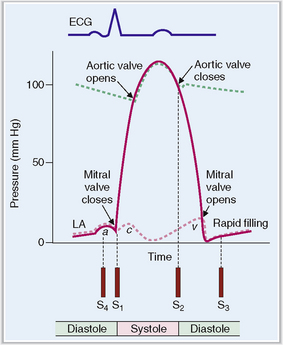
The cardiac cycle can be monitored by recording the pressure in various chambers or in the aorta, by measuring the volume of blood in various chambers, by recording the electric potential in electrocardiographic (ECG) tracings, and by monitoring the opening and closing of valves using a cardiac phonograph (Fig. 4-13).
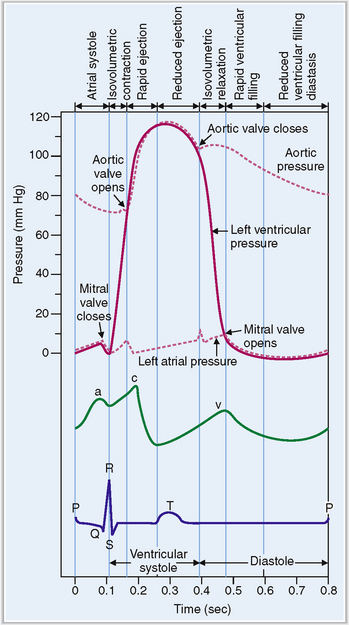
Figure 4-13 Electrical and mechanical events during the cardiac cycle.
(Modified from Guyton AC, Hall JE, Textbook of Medical Physiology, 10th ed. Saunders, Philadelphia, 2000, p. 99.)
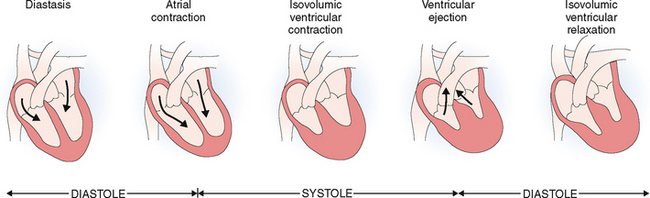
The cardiac cycle begins with the formation of an action potential inside the sinus node. This occurs during diastole when the muscle is relaxed and the ventricles dilated. Through the opening of the mitral and tricuspid valves the ventricles fill until the pressure inside the ventricles reaches the pressure in the atria. This part of the heart cycle is called diastasis. The impulses generated in the SA node are transmitted to the atrial musculature. Spreading of the depolarization through the atria is seen in ECG as the P wave. The impulses also reach the AV node from which they enter into the ventricles. The 0.10-second delay in the relay of the impulses to the atrial muscles and the entry of the electric current into the ventricles allows enough time for the atrial contraction, which typically precedes ventricular contraction before the arteriovenous (A-V) valves close.
At the end of systole the aortic valve closes and diastole begins with isovolumic relaxation of the ventricles. The closure of the aortic valve is heard as the second heart sound (S2). At the end of the isovolumic relaxation the A-V valve opens and the rapid filling (inflow) phase of diastole begins; the cycle is then repeated. The third cardiac sound (S3) can be heard during the period of rapid filling in young persons; in older persons S3 is a sign of ventricular dysfunction. The fourth cardiac sound (S4) is produced by atrial contraction. These phases of the systole and diastole are presented in Figure 4-14. Heart sounds are shown in Figure 4-15.
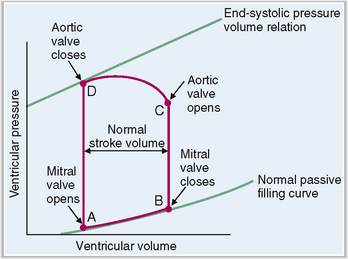
The four phases of the ventricular systole and diastole can be best presented as a pressure–volume loop.
Changes in the ratio of left ventricular volume and intraventricular pressure during the four periods of the cardiac cycle are shown in Figure 4-16. This pressure–volume loop has four parts, as follows:
 Diastolic filling. At point A the mitral valve opens, allowing the influx of blood from the atrium. The volume in the ventricle increases, but the pressure rises only minimally.
Diastolic filling. At point A the mitral valve opens, allowing the influx of blood from the atrium. The volume in the ventricle increases, but the pressure rises only minimally.
 Isovolumic contraction. At point B the mitral valve closes and the ventricle begins contracting. The pressure in the ventricle rises.
Isovolumic contraction. At point B the mitral valve closes and the ventricle begins contracting. The pressure in the ventricle rises.
 Ejection. At point C the aortic valve opens and the period of ejection of blood into the aorta begins. The intraventricular pressure continues rising but then declines slightly. The volume of blood in the left ventricle drops from 120 mL to less than 50 mL.
Ejection. At point C the aortic valve opens and the period of ejection of blood into the aorta begins. The intraventricular pressure continues rising but then declines slightly. The volume of blood in the left ventricle drops from 120 mL to less than 50 mL.
 Isovolumic relaxation. At point D the aortic valve closes and the ventricle begins relaxation. The intraventricular pressure and the volume of blood in it are low. Then the cycle repeats.
Isovolumic relaxation. At point D the aortic valve closes and the ventricle begins relaxation. The intraventricular pressure and the volume of blood in it are low. Then the cycle repeats.
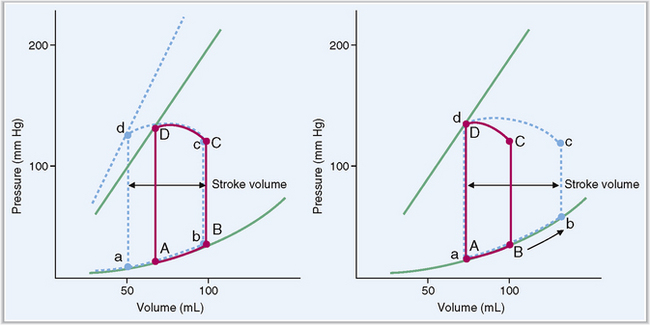
The ejection fraction is usually about 60% of the total blood volume in the ventricle.
At the end of diastole each ventricle contains approximately 110 to 120 mL of blood, known as end-diastolic volume. Stroke volume output during systole equals 70 mL, and the remaining 40 to 50 mL is called the end-systolic volume. The ejection fraction is calculated as the percentage of end-diastolic volume that is ejected from the ventricle. In normal healthy persons it is in the range of 60%. Stroke volume output can be increased during strong cardiac contractions, which reduce the end-systolic volume to 10 to 20 mL, as well as by increasing the end-diastolic volumes of the ventricle (Fig. 4-17).
Clinical and Laboratory Evaluation of Cardiac Diseases
FAMILY AND PERSONAL HISTORY
Family and personal history may provide valuable clues about the nature of cardiovascular disease (Table 4-1). Many cardiovascular diseases are multifactorial and have a tendency to affect several members of a family. Some, such as congenital heart diseases, are typically found in children; others, such as cardiomyopathies, may be diagnosed at any age. Still others, such as atherosclerosis and congestive heart failure, are more prevalent in the elderly.
Table 4-1 Risk Factors for Heart Diseases
| TYPE OF RISK FACTOR | SPECIFIC DISEASES–RISK FACTOR ASSOCIATIONS |
| Hereditary factors | |
| Social/nutritional factors | |
| Aging | Atherosclerosis: Coronary heart disease |
| Infections | |
| Medical and surgical procedures | Sepsis: Endocarditis |
| Drugs/toxins | |
| External mechanical factors |
SIGNS AND SYMPTOMS OF HEART DISEASES
Chest discomfort and pain are common symptoms that may be of cardiac or noncardiac origin.
Chest discomfort and pain are closely related sensations that vary in intensity. Minor pain may be perceived as discomfort, and pain often begins as discomfort that gradually intensifies. Clinically it is customary to subdivide chest pain into two groups: cardiac and noncardiac. The most common causes of acute chest discomfort and pain are listed in Table 4-2.
Table 4-2 Major Causes of Chest Discomfort and Pain
Cardiac pain may be classified as ischemic or pericardial.
Ischemic cardiac pain originates from the myocardium that is not receiving enough oxygen for its needs. Typically this kind of pain is characteristic of ischemic coronary heart disease and is found in patients who have angina pectoris or who have had or are having a myocardial infarction (MI). Similar pain is experienced by patients who have aortic stenosis preventing normal perfusion of the coronary arteries.
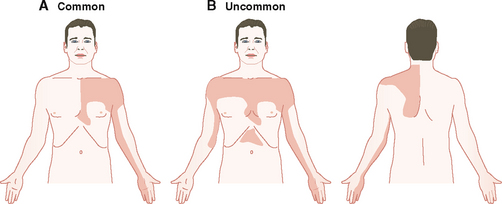
Ischemic cardiac discomfort and pain are described as heaviness or pressure or squeezing behind the sternum (retrosternal pain). The pain has an epicenter over the sternum radiating to the arm, neck, and lower jaw. Most often it radiates into the left arm along the ulnar side (Fig. 4-18). Less commonly it may be bilateral, and occasionally it may radiate to the back, usually along the left scapula. The pain is usually related to exercise, but in MI it may be of sudden onset and occur during sleep or rest. The pain of angina is usually short-lived and can be relieved by nitroglycerine, in contrast to the pain of MI, which is often crushing and of longer duration.
Pericardial pain is caused by pericarditis and is typically most intense behind the sternum or at the apex of the heart. It is described as stabbing, burning, or piercing. It lasts longer than ischemic pain and is unrelated to exercise. It may be aggravated by lying down, coughing, deep inspiration, or movement. It can be partially relieved by leaning forward or preventing chest movements. Pericardial pain is usually not radiating outside the chest.
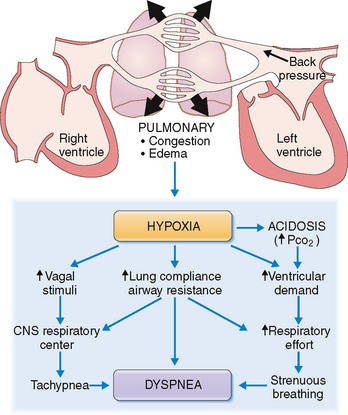
Cardiac dyspnea results from increased respiratory effort aimed at compensating for hypoxia.
An important aspect of cardiac dyspnea is the sensation of chest tightness and shortness of breath. These symptoms result from impulses emanating from mechanoreceptors in the lungs, sensory stimuli from the chest wall, and those generated by the chemoreceptors reacting to the reduced concentration of oxygen in the blood. All these afferent signals act on the respiratory center to generate an adequate ventilatory response, perceived by the individual as a “respiratory effort” or “labored breathing” (Fig. 4-19).
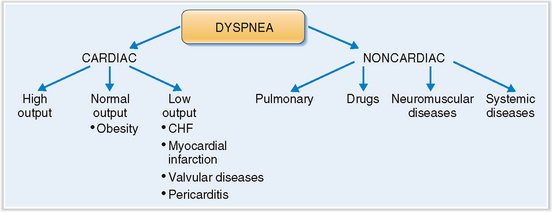
Cardiac dyspnea must be distinguished from noncardiac dyspnea.
When a patient presents with dyspnea it is most important to establish whether it is acute or chronic and to decide whether it is of cardiac or noncardiac origin. The most common form of noncardiac dyspnea is respiratory dyspnea caused by a variety of bronchopulmonary diseases, such as bronchial asthma, chronic obstructive pulmonary disease, and pneumonia (Fig. 4-20). Systemic diseases such as acidosis or neuromuscular diseases such as myasthenia gravis also may affect breathing and cause dyspnea. All these diseases can be excluded from the differential diagnosis by taking a thorough history, performing a complete physical examination, and conducting any necessary additional testing.
Cardiac dyspnea is traditionally divided into two forms: dyspnea on effort and dyspnea at rest.
 Dyspnea on effort may be the first sign of heart failure and is often associated with anginal pain. The patient typically complains of being short of breath after climbing up the stairs or strenuously walking. Usually it is progressive and can become incapacitating.
Dyspnea on effort may be the first sign of heart failure and is often associated with anginal pain. The patient typically complains of being short of breath after climbing up the stairs or strenuously walking. Usually it is progressive and can become incapacitating.
 Dyspnea at rest is a common sign of heart disease and is usually related to pulmonary congestion and edema due to left heart failure. Orthopnea is a term used to describe breathing discomfort experienced while the patient is lying down that is relieved by sitting up in bed or using several pillows. Paroxysmal nocturnal dyspnea typically wakes the patient, who has a feeling of suffocation. It may be associated with wheezing or coughing and is occasionally called “cardiac asthma.” A common cause of this form of dyspnea is tachyarrhythmia, which may be noticed by the patient. Acute heart failure, as in MI, can also cause nocturnal dyspnea.
Dyspnea at rest is a common sign of heart disease and is usually related to pulmonary congestion and edema due to left heart failure. Orthopnea is a term used to describe breathing discomfort experienced while the patient is lying down that is relieved by sitting up in bed or using several pillows. Paroxysmal nocturnal dyspnea typically wakes the patient, who has a feeling of suffocation. It may be associated with wheezing or coughing and is occasionally called “cardiac asthma.” A common cause of this form of dyspnea is tachyarrhythmia, which may be noticed by the patient. Acute heart failure, as in MI, can also cause nocturnal dyspnea.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree