Cardiac Arrest
KEY CONCEPTS
![]() High-quality cardiopulmonary resuscitation (CPR) with minimal interruptions in chest compressions should be emphasized in all patients following cardiac arrest.
High-quality cardiopulmonary resuscitation (CPR) with minimal interruptions in chest compressions should be emphasized in all patients following cardiac arrest.
![]() The AHA algorithm for basic life support following cardiac arrest now emphasizes circulation, airway, and breathing forming the pneumonic “CAB” versus the historic pneumonic “ABC.”
The AHA algorithm for basic life support following cardiac arrest now emphasizes circulation, airway, and breathing forming the pneumonic “CAB” versus the historic pneumonic “ABC.”
![]() The purpose of using vasopressor therapy following cardiac arrest is to augment low coronary and cerebral perfusion pressures encountered during CPR.
The purpose of using vasopressor therapy following cardiac arrest is to augment low coronary and cerebral perfusion pressures encountered during CPR.
![]() Despite several theoretical advantages with vasopressin, clinical trials have not consistently demonstrated superior results over that achieved with epinephrine.
Despite several theoretical advantages with vasopressin, clinical trials have not consistently demonstrated superior results over that achieved with epinephrine.
![]() Amiodarone remains the preferred antiarrhythmic during cardiac arrest with lidocaine considered as an alternative.
Amiodarone remains the preferred antiarrhythmic during cardiac arrest with lidocaine considered as an alternative.
![]() Successful treatment of both pulseless electrical activity (PEA) and asystole depends almost entirely on diagnosis of the underlying cause.
Successful treatment of both pulseless electrical activity (PEA) and asystole depends almost entirely on diagnosis of the underlying cause.
![]() Intraosseous administration is the preferred alternative route for administration if IV access cannot be achieved.
Intraosseous administration is the preferred alternative route for administration if IV access cannot be achieved.
CARDIAC ARREST
Cardiac arrest is defined as the cessation of cardiac mechanical activity as confirmed by the absence of signs of circulation (e.g., a detectable pulse, unresponsiveness, and apnea).1 While there is wide variation in the reported incidence of cardiac arrest, it is estimated that there are 350,000 people in North America each year who suffer a cardiac arrest and receive attempted resuscitation.2 Approximately half of those are in an outpatient setting. Unfortunately, survival rates have not significantly improved over 30 years, ranging between 6.7% and 8.4%, despite enormous efforts in research and development.3 Survival following in-hospital cardiac arrest is somewhat higher (approximately 18%), with higher rates being observed in victims with a shockable first documented rhythm.4
EPIDEMIOLOGY
In adult patients, cardiac arrest usually results from the development of an arrhythmia.5 Historically, ventricular fibrillation (VF) and pulseless ventricular tachycardia (PVT) have been the most common initial rhythm accounting for 40% to 60% of out-of-hospital arrests, but their incidence now is estimated to be only about 25%.2,6 In fact, one study of out-of-hospital arrests reported VF/PVT as the first recorded rhythm in only 13% of patients.7 The reason for this change has not been firmly established. Possible explanations include the influence of noncardiac causes of arrest that typically present with apnea leading to bradycardia and then pulseless electrical activity (PEA) or asystole. A second explanation is the increasing role of implantable pacemakers and defibrillators.8 Finally, it has been suggested that β-blockers and ACE inhibitors may shorten the duration of VF and the expanded use of these drug classes for ischemic heart disease and heart failure may account for the increased occurrence of non-VF/PVT rhythms.6 Nonetheless, this declining incidence is particularly concerning as survival rates are substantially higher with shockable rhythms such as VF and PVT compared with those with nonshockable rhythms such as PEA and asystole. Survival rates with VF/PVT are roughly 15% to 23% versus 0% to 5% with asystole.3
A similar finding has been observed with in-hospital cardiac arrest. One study of 411 U.S. hospitals including approximately 52,000 adult patients revealed the incidence of VF and PVT to be 7.3% and 16.8%, respectively.4 In this trial, survival rates were 37% for both VF and PVT compared with 12% (PEA) and 11% (asystole). Patients with VF/PVT were more likely to have myocardial infarction (MI) as the immediate factor prearrest, while acute respiratory failure and hypotension were the immediate factors more commonly found in patients with PEA/asystole.
In pediatric patients, cardiac arrest typically results from respiratory failure and asphyxiation. As such, the initial rhythm most often encountered in out-of-hospital arrest is PEA or asystole.9 This could explain the dismal survival rates with out-of-hospital, pediatric cardiac arrest (approximately 6%), with the lowest being observed in infants compared with children and adolescents (4%, 10%, and 13%, respectively).10 Survival following in-hospital cardiac arrest appears higher with an overall rate of 27%. In fact, children are more likely to survive in-hospital arrest versus adults and infants have a higher survival rate than children.10
ETIOLOGY
The most common clinical finding in adult patients who suffer cardiac arrest is coronary artery disease accounting for roughly 80% of sudden cardiac deaths.5 Approximately 10% to 15% of sudden cardiac deaths occur in patients with cardiomyopathies (e.g., hypertrophic cardiomyopathy, dilated cardiomyopathy), and the remaining 5% to 10% are composed of either structurally abnormal congenital cardiac conditions or patients with structurally normal but electrically abnormal heart. Unfortunately, in many patients (approximately two thirds), cardiac arrest is the first clinical sign of coronary artery disease with no preceding signs or symptoms.11
In pediatric patients, cardiac arrest is often the terminal event of respiratory failure or progressive shock.12 Out-of-hospital arrests frequently are associated with trauma, sudden infant death syndrome, drowning, poisoning, choking, severe asthma, and pneumonia. In-hospital arrests, on the other hand, are associated with sepsis, respiratory failure, drug toxicity, metabolic disorders, and arrhythmias.
PATHOPHYSIOLOGY OF CARDIAC ARREST
There are two distinctly different pathophysiologic conditions associated with cardiac arrest. The first is primary cardiac arrest whereby arterial blood is typically fully oxygenated at the time of arrest. The second is cardiac arrest secondary to respiratory failure in which lack of ventilation leads to severe hypoxemia, hypotension, and secondary cardiac arrest. It is important to understand specific condition at hand as different treatment approaches are likely necessary.13
CLINICAL PRESENTATION
Cardiac arrest is characterized by the cessation of cardiac mechanical activity; therefore, signs and symptoms are consistent with those encountered when there is no circulation. In the setting of cardiac causes of arrest, anxiety, crushing chest pain, nausea, vomiting, and diaphoresis can precede the event. Following an arrest, individuals are unresponsive, apneic, and hypotensive and do not have a detectable pulse. Extremities are cold and clammy and cyanosis is common.
TREATMENT
Cardiopulmonary resuscitation (CPR) is an attempt to restore spontaneous circulation by performing chest compressions (to restore threshold blood flows, particularly to the heart and brain) with or without ventilations. There are two proposed theories describing the mechanism of blood flow during CPR.14 The original theory is known as the cardiac pump theory and is based on the active compression of the heart between the sternum and vertebrae thereby creating forward flow. Echocardiography, however, has revealed that left ventricular size does not always change with compressions and the mitral valve may, in fact, be open.14 The second theory is the thoracic pump theory. This theory is based on intrathoracic pressure alterations induced by chest compressions and the differential compressibility of the arteries and veins. In this model, the heart merely acts as a passive conduit for flow. It is likely that both models contribute to the mechanism of blood flow with CPR.
High-quality CPR continues to be emphasized in the latest guidelines published by the American Heart Association (AHA). Clinicians must focus on proper technique, including adequate rate and depth of compressions, allowance of chest recoil after each compression, avoiding excessive ventilation, and minimizing interruptions.2 One study, in patients suffering out-of-hospital VF, reported an increased chance of survival as chest compression fraction increased (e.g., the proportion of resuscitation time without spontaneous circulation where chest compressions were administered).15 Unfortunately, the provision of high-quality CPR is frequently suboptimal particularly when rescuers become fatigued.10,16 There are several devices available that provide prompts and/or feedback in “real time” however, data illustrating improvement in survival are lacking.16 Additionally, mechanical devices designed to improve hemodynamics have been studied but inconsistent results limit their applicability in routine practice.17
Desired Outcome
The global goals of resuscitation are to preserve life, restore health, relieve suffering, limit disability, and respect the individual’s decisions, rights, and privacy.18 This can be accomplished via CPR by the return of spontaneous circulation (ROSC) with effective perfusion and ventilation as quickly as possible to minimize hypoxic damage to vital organs. Survival to hospital discharge with good neurologic function should be considered the primary treatment outcome sought by clinicians. Survival to hospital discharge in a vegetative or comatose state cannot be classified as a success and can impose a tremendous economic burden on the healthcare system. Additionally, most patients would choose not to continue living in a massively disabled state.19
The presence of a healthcare advanced directive allows patients to communicate their wishes and preferences regarding medical care and may lead to a “do not attempt resuscitation (DNAR)” order. As many cardiac arrests occur following terminal illnesses and end-of-life care, “allow natural death (AND)” has become a preferred term to replace DNAR.18 These orders should explicitly state the resuscitation interventions that are to be performed and have clearly been communicated by the patient, his or her family, or a surrogate decision maker.
General Approach to Treatment
Cardiopulmonary Resuscitation
Resuscitation techniques have been studied for many years. The first landmark article was published in 1960 and described the outcome of 20 patients who were given closed chest compressions at a rate of 60/min.20 Artificial ventilation was used to augment the compressions, and three patients were given defibrillation for VF. In this landmark article, all 20 patients had ROSC, and 14 lived for an extended period of time, with reported good neurologic status. Initial descriptions after this started to integrate the approach to cardiac arrest, including three phases.21
In 1966, the AHA first published guidelines for the treatment of cardiac arrest.22 Since then, national conferences and organized committees have played a major role in encouraging widespread competency in CPR technique. There have been tremendous revisions of the guidelines over the years, and this is true of the most recent guidelines, published in 2010.2 These guidelines continue to emphasize the “chain of survival” to highlight the treatment approach and illustrate the importance of a timely response. The updated guidelines list five links in the chain of survival:
1. Immediate recognition of cardiac arrest and activation of emergency medical services (EMS)
2. Early CPR with an emphasis on chest compressions
3. Rapid defibrillation
4. Effective advanced life support
5. Integrated postcardiac arrest care
While all five links of the chain of survival are important, the most crucial would seem to be the first three, particularly early CPR with good chest compressions.2 When used together, survival rates can approach 50% following witnessed out-of-hospital VF arrest.23 CPR provides critical blood flow to the heart and brain, prolongs the time VF is present (prior to the deterioration to asystole), and increases the likelihood that a shock will terminate VF resulting in a rhythm compatible with life.2 For every minute that elapsed from collapse to successful defibrillation during witnessed VF arrests, survival rates decrease by 7% to 10% if no CPR is provided.24 If immediate CPR is added, the decrease in survival is more gradual (down to 3% to 4% per minute postcollapse).25 In effect, CPR can increase the likelihood of survival threefold from arrest to survival. Basic CPR alone, however, is not likely to terminate VF and lead to ROSC. Thus, the 2010 AHA guidelines emphasize the integration of early CPR and defibrillation, especially mentioning the use of automatic external defibrillators.25
![]() As in the 2005 AHA guidelines for CPR and emergency cardiovascular care (ECC), the AHA continues to emphasize the provision of high-quality CPR with minimal interruptions in chest compressions. In addition, algorithms seem to be more simplified, and there is emphasis on the use of end-tidal carbon dioxide (ETCO2) to guide resuscitation.26 Furthermore, there is growing importance of postarrest care, reflecting that optimization of many organ systems may help improve outcomes.27 The use of drug therapy and airway adjuncts, on the other hand, have continued to devolve to a minimal role as survival to hospital discharge does not appear to be impacted.
As in the 2005 AHA guidelines for CPR and emergency cardiovascular care (ECC), the AHA continues to emphasize the provision of high-quality CPR with minimal interruptions in chest compressions. In addition, algorithms seem to be more simplified, and there is emphasis on the use of end-tidal carbon dioxide (ETCO2) to guide resuscitation.26 Furthermore, there is growing importance of postarrest care, reflecting that optimization of many organ systems may help improve outcomes.27 The use of drug therapy and airway adjuncts, on the other hand, have continued to devolve to a minimal role as survival to hospital discharge does not appear to be impacted.
Basic Life Support The 2010 AHA guidelines represent a paradigm shift in the provision of basic life support (BLS). Historically, BLS and advanced cardiac life support (ACLS) providers have been taught the pneumonic “ABC,” representing, respectively, airway, breathing, and circulation for the CPR sequence. ![]() The 2010 guidelines have changed this to “CAB,” or circulation, airway, and breathing.28
The 2010 guidelines have changed this to “CAB,” or circulation, airway, and breathing.28
When first encountering a victim of cardiac arrest, the initial action is to determine responsiveness of the patient. If there is no response, the rescuer should immediately activate the emergency medical response team, and obtain (or call for) an automated external defibrillator (AED) (if one is available) and then immediately start CPR with chest compressions. A true cardiac arrest victim will be unresponsive, and agonal respirations can be confused with normal breathing. Thus, the “look, listen, and feel” for respirations that has been a standard protocol for initial assessment is no longer recommended.2 Similarly, pulse recognition is often inaccurate, and it is now recommended that lay rescuers not check for a pulse. Healthcare providers should assess for a pulse but take no more than 10 seconds to do so. If one is not detected within this short time frame, then chest compressions should be initiated immediately.28,29
The prompt provision of chest compressions is thus of paramount importance, and rescuers should attempt them regardless of rescuer experience or skill level. The teaching of BLS now focuses on delivering high-quality CPR with a rate of at least 100/min, adequate depth (at least 2 in [5 cm] in an adult), allowing full chest recoil, minimizing interruptions in compressions, and avoiding excessive ventilation.
While it is true that opening the airway has the potential to improve oxygenation and allow for better attempts at ventilation, this can be very challenging, especially if the rescuer is alone and is a novice. Thus, the simplified adult BLS algorithm calls for the initiation of CPR, with rhythm check every 2 minutes, shocking if indicated, with continued repetition.
Once chest compressions have been started, it is then appropriate for a trained rescuer to attempt to deliver rescue breaths, either by mouth-to-mouth or preferentially by bag-mask ventilation. The current guidelines recommend delivering a breath over 1 second, using enough volume to elicit a visible chest rise, and using a compression-to-ventilation ratio of 30:2 for one rescuer.28
The 2010 AHA guidelines for CPR and ECC continue to stress that there should be minimal interruptions in chest compressions. If there is no AED available, then cycles of compressions/breaths should continue, with pulse checks every 2 minutes until help arrives or the patient regains spontaneous circulation. If there is an AED available, then the rhythm should be checked to determine if defibrillation is advised. If so, then one shock should be delivered with the immediate resumption of chest compressions (and rescue breaths, if being provided). After 2 minutes (five cycles of 30:2 compression-to-ventilation), the rhythm should be reevaluated to determine the need for defibrillation. This algorithm should be repeated until help arrives, or the rhythm is no longer “shockable.” If the rhythm is not shockable, then chest compressions and rescue breath cycles should be continued until help arrives, or the victim recovers spontaneous circulation (Fig. 2-1).
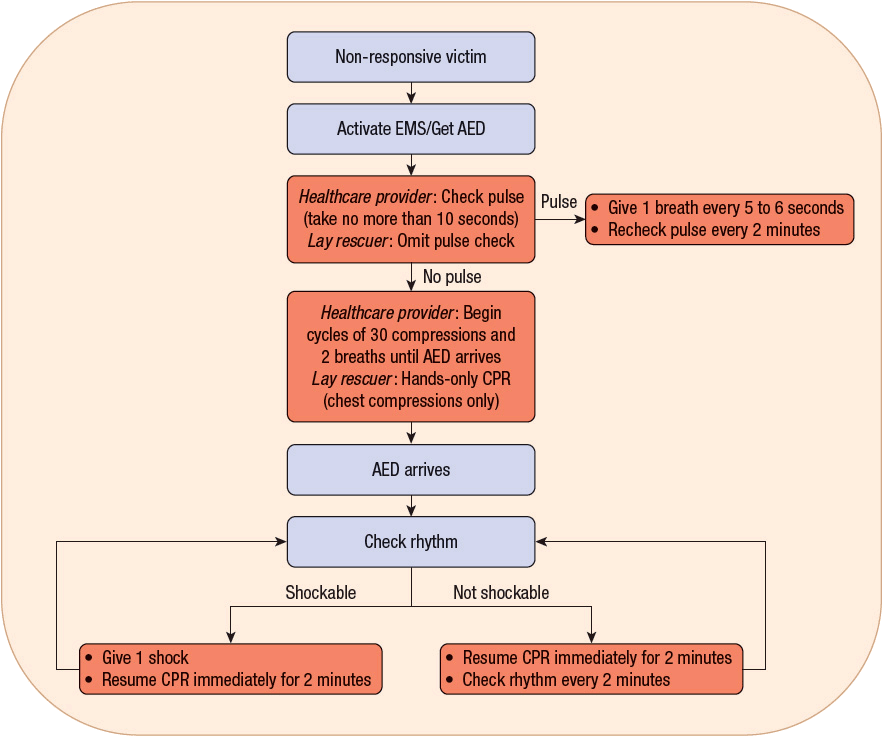
FIGURE 2-1 Treatment algorithm for adult cardiac arrest: basic life support (BLS).
Despite widespread dissemination of cardiac arrest guidelines and the ongoing education even of healthcare providers, there is ample evidence that chest compression quality remains poor in general. Furthermore, it has been reported that only 20% to 30% of adults with out-of-hospital cardiac arrest receive bystander CPR.28 This has led to further educational interventions in an attempt to increase quality of CPR, and EMS dispatchers will often attempt to give instructions over the phone when EMS is activated.
There is now a push for hands-only CPR for lay persons, given data that show similar survival compared with the addition of rescue breaths. There has been reluctance on many bystanders to consider mouth-to-mouth, although one data set cites panic as a reason not to pursue bystander CPR rather than actual reluctance.30
Advanced Cardiac Life Support Once ACLS providers arrive, then further definitive therapy is given. An advanced airway (endotracheal tube, laryngeal mask airway, or even bag-valve mask) can be utilized to provide ventilation. When this occurs, the rescuers no longer need to provide the cycles of 30:2 compression-to-ventilation. Instead, continuous chest compressions are recommended without pauses for ventilations, and the rescuer providing the ventilations needs to deliver a breath once every 6 to 8 seconds.
Monitoring during CPR has also evolved over time. Animal and human studies have shown that monitoring of ETCO2, coronary perfusion pressure (CPP), and central venous oxygen saturation (SCVO2) can provide valuable information as to the success of resuscitation.26 Surprisingly, no study has ever shown the validity of checking a pulse during ongoing CPR. ETCO2 is the concentration of carbon dioxide in exhaled air at the end of expiration. During cardiac arrest, the level of ETCO2 decreases because there is no flow through the pulmonary circulation. Thus, a persistently low ETCO2 (i.e., <10 mm Hg [<1.3 kPa]) during CPR in intubated patients suggests that ROSC is unlikely.26 In fact, the return of ETCO2 abruptly to a normal level is likely to correlate with ROSC. In patients without ROSC and persistently decreased ETCO2, it is advised to evaluate the effectiveness of CPR, since good chest compressions can increase ETCO2 somewhat. The latest guidelines convincingly recommend ETCO2 monitoring during CPR if at all possible.26
CPP and SCVO2 require more invasive monitoring and will not be covered.
If the cardiac rhythm is not deemed to be shockable, then it is likely to be either asystole or PEA (Fig. 2-2). For PEA, the rescuer must consider reversible causes. If the person is in VF or PVT, then one shock should be delivered (appropriate to the available electrical device), with the immediate resumption of chest compressions (utilizing 30 compressions to 2 breaths for 5 cycles, or 2 minutes of continuous compressions with assisted ventilations) prior to rechecking the rhythm or pulse. If there is still a shockable rhythm, then one shock should be delivered, and at this time pharmacologic intervention can be considered. After the first unsuccessful shock, vasopressors are the initially recommended pharmacologic intervention (before or after the second shock), and after the second unsuccessful shock, antiarrhythmics can be considered (before or after the third shock). Chest compressions for 2 minutes (five cycles of chest compressions-to-breaths) should be performed in between attempts at defibrillation. This algorithm will repeat until a pulse is obtained with effective circulation, the rhythm changes, or the patient expires. For completeness, please refer to the guidelines published by the AHA.26
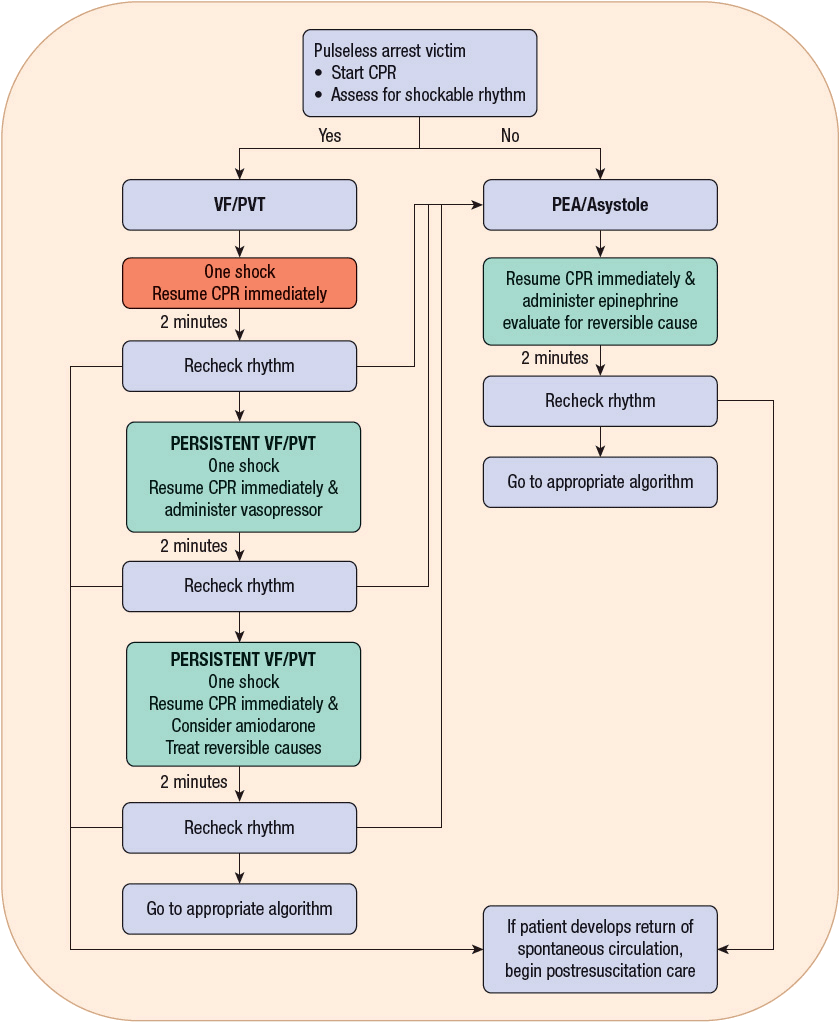
FIGURE 2-2 Treatment algorithm for adult cardiac arrest: advanced cardiac life support (ACLS).
Cardiocerebral Resuscitation
It was interesting that after the previous AHA publication of guidelines in 2005, there was very quick questioning of appropriateness. Authors at the time seemed to favor a concept known as cardiocerebral resuscitation (CCR).13 This “clarion call for change” was made in light of the suboptimal outcomes observed with the ECC guidelines as well as several limitations with the guideline process.31 CCR has been embraced by the new 2010 AHA guidelines, and consists of three major components: (a) continuous chest compressions for bystander resuscitation, (b) simplified BLS and ACLS algorithm for providers, and (c) aggressive postresuscitation care including therapeutic hypothermia and early catheterization/intervention.
CCR initially advocated continuous chest compressions without mouth-to-mouth ventilations for witnessed cardiac arrests, and has led to updated guidelines as listed above. Chest compressions deliver a small but critical amount of oxygen to the brain and myocardium. Cerebral and CPPs, however, build up slowly once chest compressions are begun. These perfusion pressures are lost if chest compressions are stopped to deliver mouth-to-mouth ventilation. In fact, in earlier studies, approximately 16 seconds were required to deliver two breaths as recommended by earlier ECC guidelines.32 The loss of perfusion during this time period has been shown to be extremely detrimental as ROSC is closely related to perfusion pressures generated during chest compressions.33
The second component of CCR is a new simplified algorithm. This protocol is based on the three-phase time-sensitive model of cardiac arrest.34 The first phase is the electrical phase (0 to 5 minutes), where prompt defibrillation is the most important intervention. The second phase is the hemodynamic phase (5 to 15 minutes), where adequate coronary and cerebral perfusion pressures, before and after defibrillation, are crucial. In fact, defibrillation prior to CPR in this phase commonly leads to asystole or PEA. This is likely due to the presence of global tissue ischemia and the need for blood flow (via chest compressions) to “flush out” deleterious metabolic factors that have accumulated during ischemia. The third phase is the metabolic phase (beyond 15 minutes) in which survival is very low and hypothermia may be the most beneficial approach.
The third component of CCR is aggressive postresuscitation care. This consists of the use of hypothermia for all comatose patients and emergent cardiac catheterization and percutaneous coronary intervention (PCI) for patients with myocardial ischemia as a potential cause of their arrest. Since its conception in 2003, clinical studies evaluating CCR have demonstrated an improvement in survival of 250% to 300% compared with conventional CPR.13
Articles are starting to appear showing that this approach is most favorable. In one study, those who received CCR had better outcomes across age groups. For those who suffered VF arrest and were under 40 years of age, the survival increased from 3.7% (standard advanced life support) to 19% (CCR patients) (odds ratio [OR] 5.94; 95% confidence interval [CI] 1.82 to 19.26).35
Ventricular Fibrillation/Pulseless Ventricular Tachycardia
Nonpharmacologic Therapy
Electrical defibrillation is the only effective method of restoring a perfusing cardiac rhythm in either VF or PVT; therefore, it is a crucial link in the “chain of survival,” especially for a witnessed arrest.25 The probability of successful defibrillation is directly related to the time interval between the onset of VF and the delivery of the first shock.25 In one study, a 23% relative improvement in survival was observed with each 1 minute reduction in the time to defibrillation (OR 0.77 [95% CI 0.73 to 0.81]).36 If fact, survival decreases an estimated 7% to 10% for each minute after arrest to defibrillation if no CPR is given.24 When bystander CPR is delivered, this decrease in survival is cut almost in half.25
Although early defibrillation is crucial for survival following cardiac arrest, several studies have suggested that CPR prior to defibrillation (consistent with the CCR model) may lead to more successful outcomes. This was reviewed extensively in the 2010 guidelines. For in-hospital cardiac arrest, if an AED is available, CPR should begin while the AED is being placed. With out-of-hospital cardiac arrest, there is growing evidence that CPR before defibrillation is, for the most part, beneficial. In studies where EMS arrivals were delayed more than 4 to 5 minutes, CPR before defibrillation increased ROSC, survival to discharge, and 1-year survival.25,37,38 In one trial, the provision of roughly 90 seconds of CPR prior to defibrillation was associated with an increased rate of hospital survival (compared with a historical control group) when response intervals were 4 minutes or longer (27% vs. 17%; P = 0.01).37 A second trial reported higher survival rates in patients with response intervals greater than 5 minutes when 3 minutes of CPR was administered prior to defibrillation (22% vs. 4%; P = 0.006).38 In a study where each defibrillation, including the first, was preceded by 200 uninterrupted chest compressions, an increase in total survival (57% [19/33] vs. 20% [18/92], P = 0.001) and neurologically normal survival (48% [16/33] vs. 15% [14/92], P = 0.001) was reported compared with standard CPR practices.39 Finally, one study noted an improvement in hospital survival (from 22% to 44%, P = 0.0024) in patients with witnessed VF using a modified resuscitation protocol that included 200 preshock chest compressions.40 In lieu of these results, the AHA guidelines continue to offer that EMS personnel may give 2 minutes of chest compressions prior to attempting defibrillation. Recommendations are similar for victims in the metabolic phase; recognizing the likelihood of achieving ROSC, however, is drastically lower.
However, as in any topic in medicine, there are data sets that can contradict standard acceptance. Koike et al. in 2011 described no better outcome with CPR before attempted defibrillation in either 1-month survival or neurologically favorable 1-month outcome.41 Thus, this is an issue of ongoing debate and study.
Clinical Controversy…
The current guidelines continue to recommend one shock for VF or PVT (as opposed to earlier iterations, where “stacked,” multiple shocks were initially given) with the immediate resumption of chest compressions.25 This revision is largely due to the prolonged time noted (approximately 55 seconds) to deliver three stacked shocks without providing adequate chest compressions.42 The defibrillation attempt should be with 360 J (monophasic defibrillator) or 150 to 200 J (biphasic defibrillator). If an AED is available, it should be used as soon as possible. However, CPR should be started immediately (after EMS activation) while the AED is being prepared. Interestingly, AEDs, which have been shown to improve survival in out-of-hospital cardiac arrest due to VF/VT, have not been shown to improve outcome following replacement of monophasic defibrillators with biphasic AEDs for in-hospital arrest.43
After defibrillation is attempted, CPR should be immediately restarted and continued for 2 minutes without checking a pulse. The omission of the pulse check after defibrillation is also a paradigm shift in the algorithm that is related to myocardial stunning with resultant poor perfusion and diminished cardiac output immediately after electrical therapy.25 After 2 minutes of chest compressions, the rhythm and pulse should be rechecked. If there is still evidence of VF or PVT, then pharmacologic therapy with repeat attempts at single-discharge defibrillation should be attempted.
Endotracheal intubation and IV access should be obtained when feasible, but not at the expense of stopping chest compressions. The 2010 AHA guidelines for CPR and ECC continue to strongly stress the need for uninterrupted CPR.28 Once an airway is achieved, patients should be ventilated with 100% oxygen. There are several airway adjuncts that are potentially available, such as laryngeal mask airways and esophageal–tracheal combination tubes. However, the definitive airway is an endotracheal tube placed with direct laryngoscopy.
Other interventions are also being evaluated as nonpharmacologic therapy. In a porcine model of VF arrest, a percutaneously placed left ventricular assist device (LVAD) was shown to sustain vital organ perfusion.44 As well, the performance of angiography and PCI during suspected MI has been studied both in animals and anecdotally in humans refractory to traditional ACLS protocol without ROSC. A review of this topic suggests that this intervention is feasible and that further investigation is warranted.45 Extracorporeal membrane oxygenation (ECMO) has also been evaluated and has been shown to improve outcomes in some series, but the logistics of widespread implementation is daunting.46 While there are no conclusive human data regarding these issues, they do raise interesting concepts to deliberate and to research.
Pharmacologic Therapy
Sympathomimetics Sympathomimetics continue to be the first pharmacologic agents administered in the setting of cardiac arrest despite limited evidence demonstrating their ability to increase neurologically intact survival to hospital discharge. Nevertheless, sympathomimetics have been associated with an increased rate of ROSC and play a major role in the pharmacotherapy of cardiac arrest.
![]() The primary goal of sympathomimetic therapy is to augment low coronary and cerebral perfusion pressures encountered during CPR. Chest compressions (via CPR) can provide some degree of blood flow to the heart and the brain but it is only about 25% of that encountered under basal conditions.47 In fact, even with properly performed chest compressions, CPPs are only 10 to 15 mm Hg and systolic arterial pressure is rarely above 80 mm Hg.48 Clinical data have indicated that ROSC is unlikely when CPP is less than 15 mm Hg and animal studies have demonstrated higher rates of ROSC when CPP was 31 mm Hg versus 14 mm Hg.49,50 Sympathomimetics therefore work to increase these pressures through their vasoconstrictive properties.
The primary goal of sympathomimetic therapy is to augment low coronary and cerebral perfusion pressures encountered during CPR. Chest compressions (via CPR) can provide some degree of blood flow to the heart and the brain but it is only about 25% of that encountered under basal conditions.47 In fact, even with properly performed chest compressions, CPPs are only 10 to 15 mm Hg and systolic arterial pressure is rarely above 80 mm Hg.48 Clinical data have indicated that ROSC is unlikely when CPP is less than 15 mm Hg and animal studies have demonstrated higher rates of ROSC when CPP was 31 mm Hg versus 14 mm Hg.49,50 Sympathomimetics therefore work to increase these pressures through their vasoconstrictive properties.
Epinephrine continues to be a drug of first choice for the treatment of VF, PVT, asystole, and PEA. It is an α– and β-receptor agonist causing both vasoconstriction and increased inotropic/chronotropic activity on the heart. Its effectiveness however is primarily through its α effects, particularly α2-activity.51
There are few prospective data evaluating epinephrine in the setting of out-of-hospital cardiac arrest. In one study, patients were randomized to receive standard ACLS with IV drug administration or standard ACLS without IV drug administration.52 There were 851 patients analyzed and VF/PVT was the initial rhythm in 34%. IV medications administered included epinephrine (79%), atropine (46%), and amiodarone (17%). A significant increase in ROSC (40% vs. 25%, P <0.001) and hospital admission (43% vs. 29%, P <0.001) was noted in patients who received IV therapy. This difference was primarily observed in patients with initial rhythms other than VF/PVT. The role of epinephrine (vs. other IV medications) in the contribution of these outcomes was not assessed. A second randomized controlled trial compared epinephrine with placebo in 534 patients.53 VF or PVT was the initial rhythm in 44% and 48% of patients in the epinephrine and placebo groups, respectively. ROSC (23.5% vs. 8.4%, P <0.001) and survival to hospital admission (25.4% vs. 13%, P <0.001) were significantly higher with epinephrine, but there was no difference in survival to hospital discharge (4% vs. 1.9%, P = 0.15). While epinephrine was effective in achieving ROSC in both shockable (OR [95% CI] = 2.5 [1.2 to 4.5]) and nonshockable (OR [95% CI] = 6.9 [2.6 to 18.4]) rhythms, its effect was more pronounced in the latter cohort. In contrast, one large prospective registry study of over 400,000 patients failed to demonstrate a survival benefit with prehospital administration of epinephrine.54 Despite a significant improvement in ROSC with epinephrine (adjusted OR [95% CI] = 2.36 [2.22 to 2.5]), both 1-month survival (adjusted OR [95% CI] = 0.46 [0.42 to 0.51]) and survival with good neurologic function (adjusted OR [95% CI] = 0.31 [0.26 to 0.36]) were lower in patients who received epinephrine. These findings were confirmed through various sensitivity analyses accounting for in-hospital epinephrine use and CPR duration. Given the disparate results with epinephrine in clinical trials, it can be considered both a cure and a curse in cardiac arrest. One potential approach, which will require validation through clinical trials, is to administer epinephrine only in settings where aortic diastolic pressure is low (i.e., less than 30 to 40 mm Hg) recognizing that a vasoconstrictive agent will provide minimal benefit (and possible harm) when perfusion pressures are adequate.55
Clinical Controversy…
One possible explanation for the negative effects of epinephrine is related to its mechanism of action. Epinephrine causes α-mediated vasoconstriction that increases coronary perfusion but can decrease perfusion to other vital organs. In fact, animal research has linked epinephrine to a decrease in cerebral microvascular blood flow and increase in brain tissue ischemia during and after CPR.56 Epinephrine also stimulates β-receptors that can increase myocardial oxygen demand, impair lactate clearance, and advance the severity of postresuscitation myocardial dysfunction.57 This has led some investigators to evaluate simultaneous adrenergic antagonist administration in conjunction with epinephrine therapy (thereby isolating the α2 effects) using an animal model.58 This approach has not been extensively studied in humans.
Several studies have compared epinephrine with other adrenergic agonists such as pure α1-agonists (phenylephrine and methoxamine) and agents with more potent α-activity (norepinephrine).59 When compared with pure α1-agonists, no advantage in long-term survival could be reported. One potential reason could be the potent α2 effects with epinephrine and the fact that these receptors lie extrajunctionally in the intima of the blood vessels making them more accessible to circulating catecholamines.60 Furthermore, during ischemia, the number of postsynaptic α1-receptors decreases, which suggests a greater role for α2-agonists during CPR.61 Epinephrine has also been compared with norepinephrine, a potent α-agonist (both α1 and α2) with some β1 effects. In the only large-scale randomized, double-blind, prospective trial in out-of-hospital cardiac arrest, there were no significant differences in ROSC, hospital admission, or discharge.62 A second, smaller study demonstrated higher resuscitation rates with norepinephrine compared with those with epinephrine (64% vs. 32%) but no significant difference in hospital discharge.63 Since the use of epinephrine has been established for many decades in evidence-based guidelines, strong outcome-related data (e.g., survival to hospital discharge) would be required for an alternative to replace it. Consequently, epinephrine remains the first-line sympathomimetic for CPR.
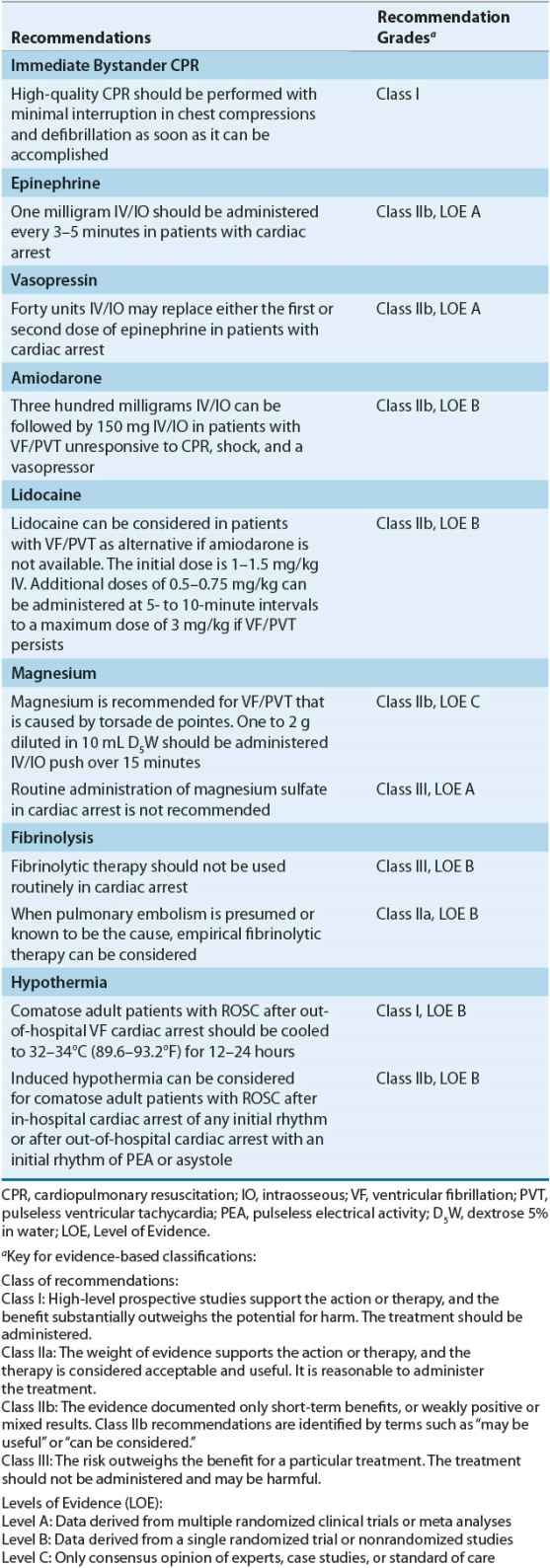
The recommended dose for epinephrine is 1 mg administered by IV or intraosseous (IO) injection every 3 to 5 minutes26 (Table 2-1). Higher doses may be administered to treat specific disorders such as β-blocker or calcium channel blocker overdose. Additionally, higher doses can be considered if indicated through arterial diastolic pressure (or CPP) monitoring. The recommended dose for epinephrine was derived from animal studies (0.1 mg/kg in a 10-kg dog) and equates to approximately 0.015 mg/kg for a 70-kg human.64 Both animal and human studies have demonstrated a positive dose–response relationship with epinephrine suggesting that higher doses might be necessary to improve hemodynamics and achieve successful resuscitation.59 These results, however, have not been replicated in human studies. In fact, some studies have reported increased morbidity with high epinephrine doses, indicative of catecholamine toxicity, including decreased cardiac indices, left ventricular dysfunction, and decreased oxygen consumption and delivery. This discrepancy between animal and human studies could be related to most victims of cardiac arrest having coronary artery disease, which is not encountered in an animal model. Additionally, atherosclerotic plaques (in humans) can aggravate the balance between myocardial oxygen supply and demand and the interval from arrest to treatment is longer in human studies than that encountered in an animal model.
TABLE 2-1 Evidence-Based Recommendations
Vasopressin Vasopressin, also known as antidiuretic hormone, is a potent, nonadrenergic vasoconstrictor that increases blood pressure and systemic vascular resistance. Although it acts on various receptors throughout the body, its vasoconstrictive properties are due primarily to its effects on the V1 receptor. Measurement of vasopressin levels in patients undergoing CPR has shown a high correlation between the levels of endogenous vasopressin released and the potential for ROSC.65 In fact, in one study, plasma vasopressin concentrations were approximately three times as high in survivors compared with those in nonsurvivors, suggesting that vasopressin is released as an adjunct vasopressor to epinephrine in life-threatening events such as cardiac arrest.66
Vasopressin may have several advantages over epinephrine. First, the metabolic acidosis that frequently accompanies cardiac arrest can blunt the vasoconstrictive effect of adrenergic agents such as epinephrine. This effect does not occur with vasopressin. Second, the stimulation of β-receptors caused by epinephrine can increase myocardial oxygen demand and complicate the postresuscitative phase of CPR. Because vasopressin does not act on β-receptors, this effect does not occur with its use. Vasopressin also may have a beneficial effect on renal blood flow by stimulating V2 receptors in the kidney, causing vasodilation and increased water reabsorption. With regard to splanchnic blood flow, however, vasopressin has a detrimental effect when compared with epinephrine.65
Despite these theoretical advantages with vasopressin, clinical trials have not consistently demonstrated superior results over that achieved with epinephrine (Table 2-2). In one large trial of out-of-hospital arrest, no significant differences were noted in ROSC, hospital admission rate, or discharge rate.69 Although when patients were stratified according to their initial rhythm, patients with asystole had a significantly higher rate of hospital admission (29% vs. 20%; P = 0.02) and discharge (4.7% vs. 1.5%; P = 0.04) with vasopressin compared with that with epinephrine. In addition, a subgroup analysis of 732 patients who required additional epinephrine therapy despite the two doses of study drug revealed significant benefits in ROSC (37% vs. 26%; P = 0.002), hospital admission rate (26% vs. 16%; P = 0.002), and discharge rate (6.2% vs. 1.7%; P = 0.002) with vasopressin. There was a trend, however, toward a poorer neurologic state or coma among the patients who survived to discharge and received vasopressin.
TABLE 2-2 Prospective Randomized Controlled Trials with Vasopressin in Cardiac Arrest
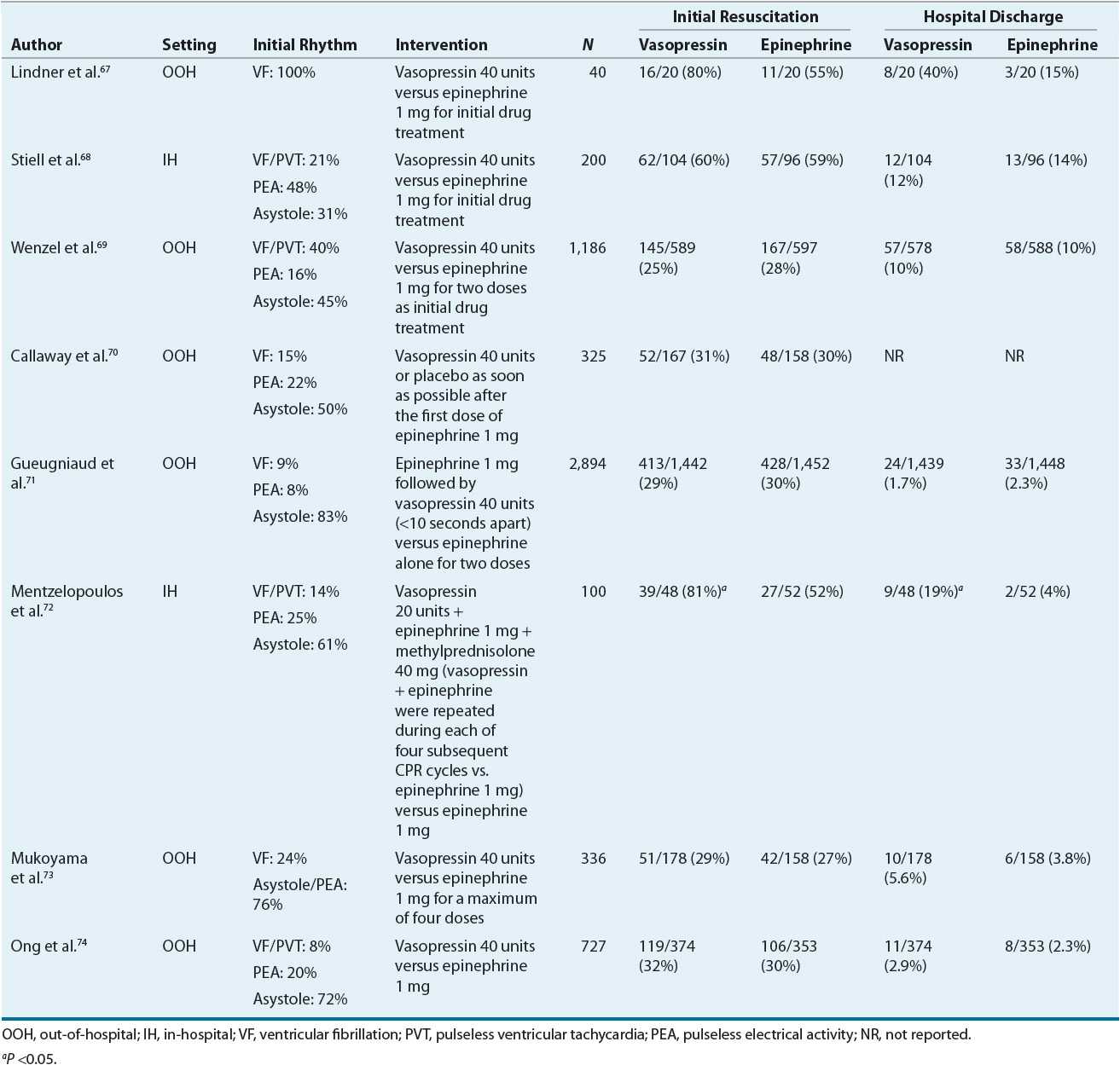
The favorable results observed in the subgroup analysis led to a prospective study evaluating the combination of vasopressin and epinephrine versus epinephrine alone.71 In this study, patients were randomized to receive either 1 mg of epinephrine followed by 40 units of vasopressin (in less than 10 seconds) or 1 mg of epinephrine plus saline placebo. Unfortunately, there were no significant differences between the combination therapy group and epinephrine-only group in any of the outcome measures studied (ROSC, survival to hospital admission, survival to hospital discharge, 1-year survival, and good neurologic recovery at discharge). In contrast, a post hoc subgroup analysis revealed a lower rate of survival (0% vs. 5.8%, P = 0.02) with combination therapy when the initial rhythm was PEA.
One study evaluated a multidrug regimen that also included corticosteroids for patients with in-hospital cardiac arrest.72 In this study, patients were randomized to receive either epinephrine alone or 20 units of vasopressin plus 1 mg of epinephrine and 40 mg of methylprednisolone (followed by hydrocortisone in the postresuscitative phase). Vasopressin 20 units plus epinephrine 1 mg was repeated during each of four subsequent CPR cycles. This study marks the first to include corticosteroids as part of drug therapy during CPR. The rationale is based on the hemodynamic effects of steroids alone with their potential to impact the intensity of the postresuscitation systemic inflammatory response and organ dysfunction. Significant benefits were observed in ROSC (81% vs. 52%, P = 0.003) and survival to hospital discharge (19% vs. 4%, P = 0.02) with combination therapy including corticosteroids. Future studies are required to determine the role of vasopressin and corticosteroids for cardiac arrest.
In lieu of the conflicting results across numerous randomized controlled trials, a meta analysis was performed to further define the role of vasopressin.75 Six studies were chosen for analysis (4, out-of-hospital arrest; 2, in-hospital arrest) including 4,745 patients. No significant improvements were noted with vasopressin therapy in ROSC (OR [95% CI] = 1.25 [0.9 to 1.74]), long-term survival (OR [95% CI] = 1.13 [0.71 to 1.78]), or favorable neurologic outcome (OR [95% CI] = 0.87 [0.49 to 1.52]). When patients were stratified based on the presence of VF/PVT as their initial rhythm, the incidence of ROSC (OR [95% CI] = 1.18 [0.82 to 1.69]) and long-term survival (OR [95% CI] = 0.95 [0.66 to 1.37]) were similar with vasopressin. Interestingly, in patients with asystole, vasopressin was associated with superior long-term survival rates relative to control (OR [95% CI] = 1.8 [1.04 to 3.12]).
![]() In summary, vasopressin appears to offer no benefit over epinephrine when used as an alternative to or when coadministered with epinephrine. Future prospective trials are needed to validate the role of vasopressin in certain subpopulations (e.g., asystole) or when combined with corticosteroids. The current recommendations for vasopressin are that one dose (40 units) administered IV/IO may replace either the first or second dose of epinephrine in the treatment of cardiac arrest.
In summary, vasopressin appears to offer no benefit over epinephrine when used as an alternative to or when coadministered with epinephrine. Future prospective trials are needed to validate the role of vasopressin in certain subpopulations (e.g., asystole) or when combined with corticosteroids. The current recommendations for vasopressin are that one dose (40 units) administered IV/IO may replace either the first or second dose of epinephrine in the treatment of cardiac arrest.



