Carcinoma with Metaplasia
The term metaplastic carcinoma has traditionally been reserved for carcinomas that exhibit microscopic structural features that diverge from glandular differentiation. In the breast, these phenotypic structural alterations are the expression of genotypic properties not manifested by normal mammary epithelium. Therefore, metaplastic carcinoma represents patterns of gene expression rather than histogenesis. This conclusion is supported by the finding of the same p53 point mutation in several components of a metaplastic carcinoma that was studied by Wang et al. (1).
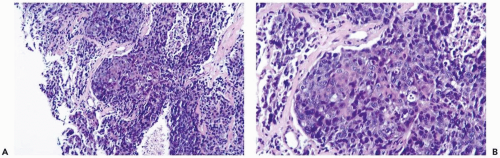
The frequency of metaplastic change in mammary carcinoma is probably underreported because inconspicuous foci are easily overlooked or ignored (Fig. 13.1). Squamous metaplasia was present in 3.7% of 1,665 invasive carcinomas reviewed by Fisher et al. (2). Heterologous or sarcomatoid metaplasia was detected in 26 of 12,045 (0.2%) breast carcinomas in another study (3). Carcinomas with metaplasia usually have low levels of estrogen receptor and are classified as receptor negative when studied by biochemistry or immunohistochemistry (4,5,6).
The range of age at diagnosis and the clinical features of metaplastic mammary carcinoma are not appreciably different from those of invasive mammary duct carcinoma (3,6). The first symptom is typically a palpable tumor. The patient usually describes rapid growth and short duration prior to diagnosis (6). The radiologic appearance of metaplastic carcinoma is not specific, except that bone formation in tumors with osseous metaplasia may be detectable by mammography (7). The tumors tend to have circumscribed contours radiologically and grossly. Velasco et al. (8) reported that metaplastic carcinomas had spiculated contours and variable signal intensity on T2-weighted MRI images. The mean or median size (3-4 cm) reported in various series tends to be greater than that of ordinary infiltrating duct carcinomas.
The cell type that gives rise to metaplastic carcinomas remains uncertain. The concurrent presence of ordinary intraductal and invasive duct carcinoma in some of these tumors and transitions observed from carcinomatous foci to metaplastic components has led to the conclusion that these neoplasms are carcinomas derived from mammary glandular epithelial cells. Immunohistochemical studies that revealed coexpression of S-100, vimentin, and cytokeratin in components with epithelial and sarcomatoid phenotypes were interpreted as evidence for the epithelial origin of both elements by some investigators (9). Others pointed to these same observations as suggesting myoepithelial origin (4).
Recent studies that used newly developed markers suggest that the sarcomatoid components of metaplastic carcinomas derive from myoepithelial cells. p63, a p53 homologue, found in the nuclei of myoepithelial and squamous cells and only rarely in glandular epithelial cells (10,11), is expressed in almost all sarcomatoid metaplastic carcinomas of the breast (11,12,13). Koker and Kleer (13) detected p63 expression in 100% of spindle and squamous metaplastic carcinomas and in one of three tumors with chondroid differentiation. In the same study, p63 was expressed in 1 of 174 (0.6%) nonmetaplastic carcinomas. There was no p63 reactivity in sarcomas or the stroma of phyllodes tumors. Other myoepithelial-associated markers, such as CK5, CD10, myosin, and maspin, as well as smooth muscle actin (SMA), are also expressed in sarcomatoid components of many of these tumors but less consistently than p63 (14,15,16). Absence of p63 reactivity in the stroma of phyllodes tumors, almost all stromal sarcomas, fibromatosis, and myofibroblastic tumors is very useful for distinguishing these lesions from metaplastic carcinoma. At present, it appears likely that tumors included in the category of metaplastic carcinoma may be a heterogeneous group of neoplasms that originate solely from the epithelium, from the myoepithelium or from both cell types. When distinct intraductal carcinoma or invasive ductal adenocarcinoma elements are not evident and the tumor is diffusely p63 positive, myoepithelial histogenesis is likely.
It has been customary to subdivide metaplastic carcinomas into two categories based on histologic constituents: squamous and heterologous or pseudosarcomatous. These
distinctions are somewhat arbitrary because some tumors exhibit both types of growth. A common pattern of metaplastic carcinoma is focal squamous metaplasia in an otherwise typical invasive duct carcinoma. A spectrum of differentiation may be found in squamous metaplasia. Mature keratinizing epithelium, sometimes with keratohyalin granules, may be associated with poorly differentiated carcinoma or spindle cell, pseudosarcomatous areas (Fig. 13.2). Spindle cell carcinoma of the breast is a subset of carcinomas with squamous metaplasia in which most or virtually all of the neoplasm has assumed a pseudosarcomatous growth pattern that resembles fibrosarcoma or fibromatosis (6,17) (Fig. 13.3). In some instances, the nature of the lesion can be obscured by an inflammatory reaction that may suggest a nonneoplastic condition, such as inflammatory pseudotumor or fasciitis (Fig. 13.4). Another variant is characterized by dense, keloid-like areas of fibrosis in which the spindle cells and stroma often have a storiform pattern (Fig. 13.5).
distinctions are somewhat arbitrary because some tumors exhibit both types of growth. A common pattern of metaplastic carcinoma is focal squamous metaplasia in an otherwise typical invasive duct carcinoma. A spectrum of differentiation may be found in squamous metaplasia. Mature keratinizing epithelium, sometimes with keratohyalin granules, may be associated with poorly differentiated carcinoma or spindle cell, pseudosarcomatous areas (Fig. 13.2). Spindle cell carcinoma of the breast is a subset of carcinomas with squamous metaplasia in which most or virtually all of the neoplasm has assumed a pseudosarcomatous growth pattern that resembles fibrosarcoma or fibromatosis (6,17) (Fig. 13.3). In some instances, the nature of the lesion can be obscured by an inflammatory reaction that may suggest a nonneoplastic condition, such as inflammatory pseudotumor or fasciitis (Fig. 13.4). Another variant is characterized by dense, keloid-like areas of fibrosis in which the spindle cells and stroma often have a storiform pattern (Fig. 13.5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree