14
Carbohydrates of Physiologic Significance
David A. Bender, PhD & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Explain what is meant by the terms monosaccharide, disaccharide, oligosaccharide and polysaccharide.
Explain what is meant by the terms monosaccharide, disaccharide, oligosaccharide and polysaccharide.
![]() Explain the different ways in which the structures of glucose and other monosaccharides can be represented, and describe the various types of isomerism of sugars and the pyranose and furanose ring structures.
Explain the different ways in which the structures of glucose and other monosaccharides can be represented, and describe the various types of isomerism of sugars and the pyranose and furanose ring structures.
![]() Describe the formation of glycosides and the structures of the important disaccharides and polysaccharides.
Describe the formation of glycosides and the structures of the important disaccharides and polysaccharides.
![]() Explain what is meant by the glycemic index of a carbohydrate.
Explain what is meant by the glycemic index of a carbohydrate.
![]() Describe the roles of carbohydrates in cell membranes and lipoproteins.
Describe the roles of carbohydrates in cell membranes and lipoproteins.
BIOMEDICAL IMPORTANCE
Carbohydrates are widely distributed in plants and animals; they have important structural and metabolic roles. In plants, glucose is synthesized from carbon dioxide and water by photosynthesis and stored as starch or used to synthesize the cellulose of the plant cell walls. Animals can synthesize carbohydrates from amino acids, but most are derived ultimately from plants. Glucose is the most important carbohydrate; most dietary carbohydrate is absorbed into the bloodstream as glucose formed by hydrolysis of dietary starch and disaccharides, and other sugars are converted to glucose in the liver. Glucose is the major metabolic fuel of mammals (except ruminants) and a universal fuel of the fetus. It is the precursor for synthesis of all the other carbohydrates in the body, including glycogen for storage; ribose and deoxyribose in nucleic acids; galactose for synthesis of lactose in milk, in glycolipids, and in combination with protein in glycoproteins and proteoglycans. Diseases associated with carbohydrate metabolism include diabetes mellitus, galactosemia, glycogen storage diseases, and lactose intolerance.
CARBOHYDRATES ARE ALDEHYDE OR KETONE DERIVATIVES OF POLYHYDRIC ALCOHOLS
Carbohydrates are classified as follows:
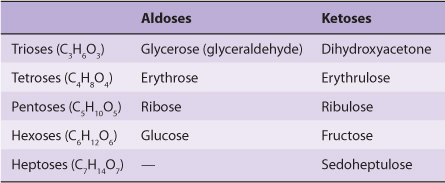
1. Monosaccharides are those sugars that cannot be hydrolyzed into simpler carbohydrates. They may be classified as trioses, tetroses, pentoses, hexoses, or heptoses, depending upon the number of carbon atoms (3-7), and as aldoses or ketoses, depending upon whether they have an aldehyde or ketone group. Examples are listed in Table 14-1. In addition to aldehydes and ketones, the polyhydric alcohols (sugar alcohols or polyols), in which the aldehyde or ketone group has been reduced to an alcohol group, also occur naturally in foods. They are synthesized by reduction of monosaccharides for use in the manufacture of foods for weight reduction and for diabetics. They are poorly absorbed, and have about half the energy yield of sugars.
TABLE 14–1 Classification of Important Sugars
2. Disaccharides are condensation products of two monosaccharide units, for example, lactose, maltose, sucrose, and trehalose.
3. Oligosaccharides are condensation products of three to ten monosaccharides. Most are not digested by human enzymes.
4. Polysaccharides are condensation products of more than ten monosaccharide units; examples are the starches and dextrins, which may be linear or branched polymers. Polysaccharides are sometimes classified as hexosans or pentosans, depending on the identity of the constituent monosaccharides (hexoses and pentoses, respectively). In addition to starches and dextrins, foods contain a wide variety of other polysaccharides that are collectively known as nonstarch polysaccharides; they are not digested by human enzymes, and are the major component of dietary fiber. Examples are cellulose from plant cell walls (a glucose polymer) and inulin, the storage carbohydrate in some plants (a fructose polymer).
BIOMEDICALLY, GLUCOSE IS THE MOST IMPORTANT MONOSACCHARIDE
The Structure of Glucose Can Be Represented in Three Ways
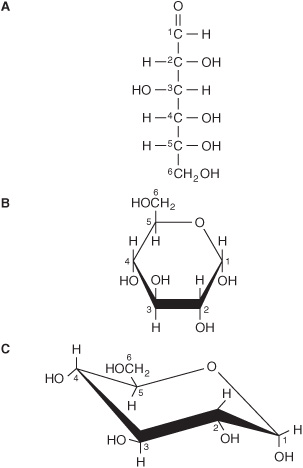
The straight-chain structural formula (aldohexose; Figure 14–1A) can account for some of the properties of glucose, but a cyclic structure (a hemiacetal formed by reaction between the aldehyde group and a hydroxyl group) is thermodynamically favored and accounts for other properties. The cyclic structure is normally drawn as shown in Figure 14–1B, the Haworth projection, in which the molecule is viewed from the side and above the plane of the ring; the bonds nearest to the viewer are bold and thickened, and the hydroxyl groups are above or below the plane of the ring. The six-membered ring containing one oxygen atom is actually in the form of a chair (Figure 14–1C).
FIGURE 14–1 D-Glucose. (A) Straight-chain form. (B) α-D-glucose; Haworth projection. (C) α-D-glucose; chair form.
Sugars Exhibit Various Forms of Isomerism
Glucose, with four asymmetric carbon atoms, can form 16 isomers. The more important types of isomerism found with glucose are as follows.
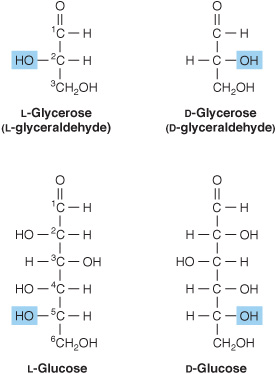
1. D and L isomerism: The designation of a sugar isomer as the D form or of its mirror image as the L form is determined by its spatial relationship to the parent compound of the carbohydrates, the three-carbon sugar glycerose (glyceraldehyde). The L and D forms of this sugar, and of glucose, are shown in Figure 14–2. The orientation of the—H and—OH groups around the carbon atom adjacent to the terminal primary alcohol carbon (carbon 5 in glucose) determines whether the sugar belongs to the Dor Lseries. When the—OH group on this carbon is on the right (as seen in Figure 14–2), the sugar is the Disomer; when it is on the left, it is the Lisomer. Most of the naturally occurring monosaccharides are Dsugars, and the enzymes responsible for their metabolism are specific for this configuration.
FIGURE 14–2 D-and L-isomerism of glycerose and glucose.
The presence of asymmetric carbon atoms also confers optical activity on the compound. When a beam of planepolarized light is passed through a solution of an optical isomer, it rotates either to the right, dextrorotatory (+), or to the left, levorotatory (-). The direction of rotation of polarized light is independent of the stereochemistry of the sugar, so it may be designated D(-), D(+), L(-), or For example, the naturally occurring form of fructose is the D(-) isomer. In solution, glucose is dextrorotatory, and glucose solutions are sometimes known as dextrose.
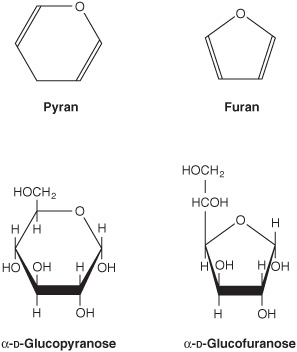
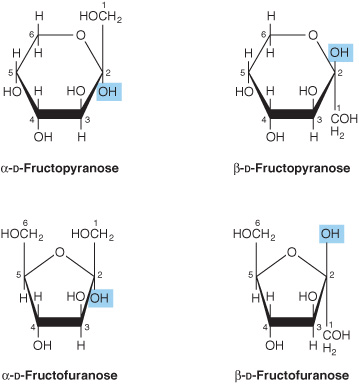
2. Pyranose and furanose ring structures: The ring structures of monosaccharides are similar to the ring structures of either pyran (a six-membered ring) or furan (a five-membered ring) (Figures 14-3 and 14-4). For glucose in solution, more than 99% is in the pyranose form.
FIGURE 14–3 Pyranose and furanose forms of glucose.
FIGURE 14–4 Pyranose and furanose forms of fructose.
3. Alpha and beta anomers:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree