PART 7: Oncology and Hematology
SECTION 1 | NEOPLASTIC DISORDERS |
99 | Approach to the Patient with Cancer |
The application of current treatment techniques (surgery, radiation therapy, chemotherapy, and biologic therapy) results in the cure of nearly two of three patients diagnosed with cancer. Nevertheless, patients experience the diagnosis of cancer as one of the most traumatic and revolutionary events that has ever happened to them. Independent of prognosis, the diagnosis brings with it a change in a person’s self-image and in his or her role in the home and workplace. The prognosis of a person who has just been found to have pancreatic cancer is the same as the prognosis of the person with aortic stenosis who develops the first symptoms of congestive heart failure (median survival, ~8 months). However, the patient with heart disease may remain functional and maintain a self-image as a fully intact person with just a malfunctioning part, a diseased organ (“a bum ticker”). By contrast, the patient with pancreatic cancer has a completely altered self-image and is viewed differently by family and anyone who knows the diagnosis. He or she is being attacked and invaded by a disease that could be anywhere in the body. Every ache or pain takes on desperate significance. Cancer is an exception to the coordinated interaction among cells and organs. In general, the cells of a multicellular organism are programmed for collaboration. Many diseases occur because the specialized cells fail to perform their assigned task. Cancer takes this malfunction one step further. Not only is there a failure of the cancer cell to maintain its specialized function, but it also strikes out on its own; the cancer cell competes to survive using natural mutability and natural selection to seek advantage over normal cells in a recapitulation of evolution. One consequence of the traitorous behavior of cancer cells is that the patient feels betrayed by his or her body. The cancer patient feels that he or she, and not just a body part, is diseased.
THE MAGNITUDE OF THE PROBLEM
No nationwide cancer registry exists; therefore, the incidence of cancer is estimated on the basis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, which tabulates cancer incidence and death figures from 13 sites, accounting for about 10% of the U.S. population, and from population data from the U.S. Census Bureau. In 2014, 1.665 million new cases of invasive cancer (855,220 men, 810,320 women) were diagnosed, and 585,720 persons (310,010 men, 275,710 women) died from cancer. The percent distribution of new cancer cases and cancer deaths by site for men and women is shown in Table 99-1. Cancer incidence has been declining by about 2% each year since 1992. Cancer is the cause of one in four deaths in the United States.
DISTRIBUTION OF CANCER INCIDENCE AND DEATHS FOR 2014 |
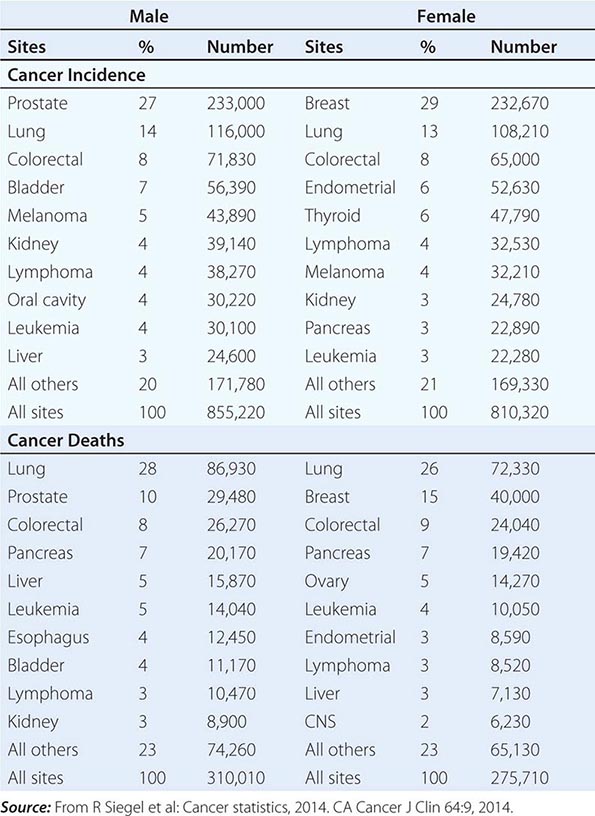
The most significant risk factor for cancer overall is age; two-thirds of all cases were in those older than age 65 years. Cancer incidence increases as the third, fourth, or fifth power of age in different sites. For the interval between birth and age 49 years, 1 in 29 men and 1 in 19 women will develop cancer; for the interval between ages 50 and 59 years, 1 in 15 men and 1 in 17 women will develop cancer; for the interval between ages 60 and 69 years, 1 in 6 men and 1 in 10 women will develop cancer; and for people age 70 and older, 1 in 3 men and 1 in 4 women will develop cancer. Overall, men have a 44% risk of developing cancer at some time during their lives; women have a 38% lifetime risk.
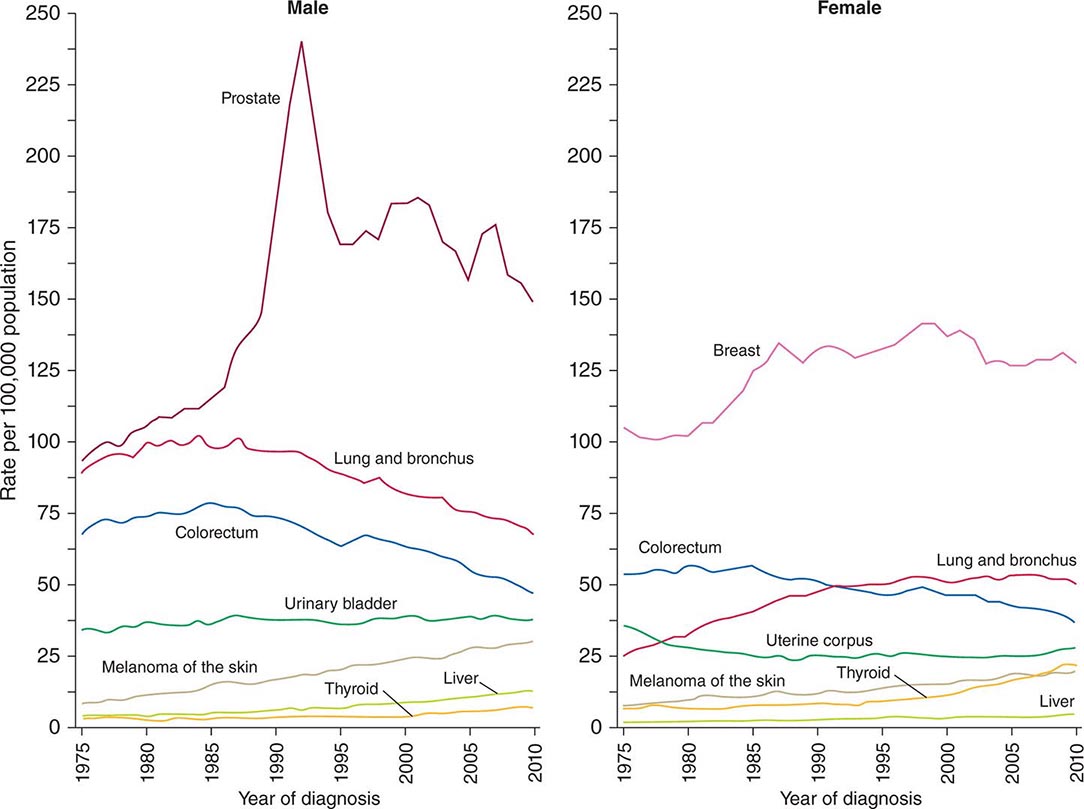
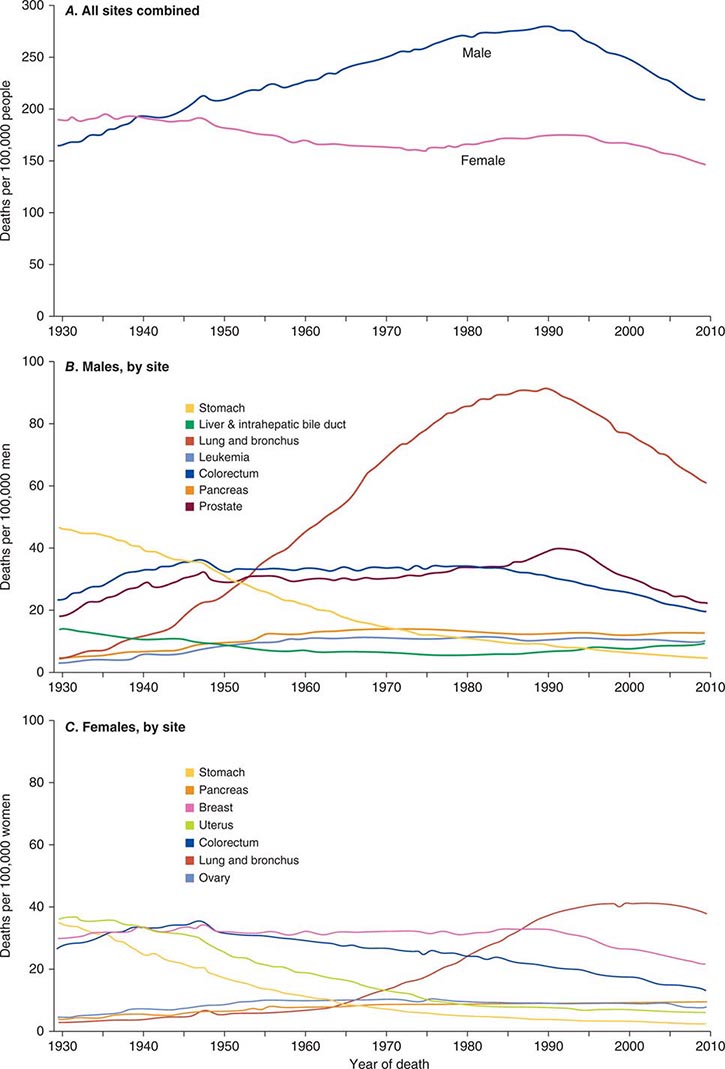
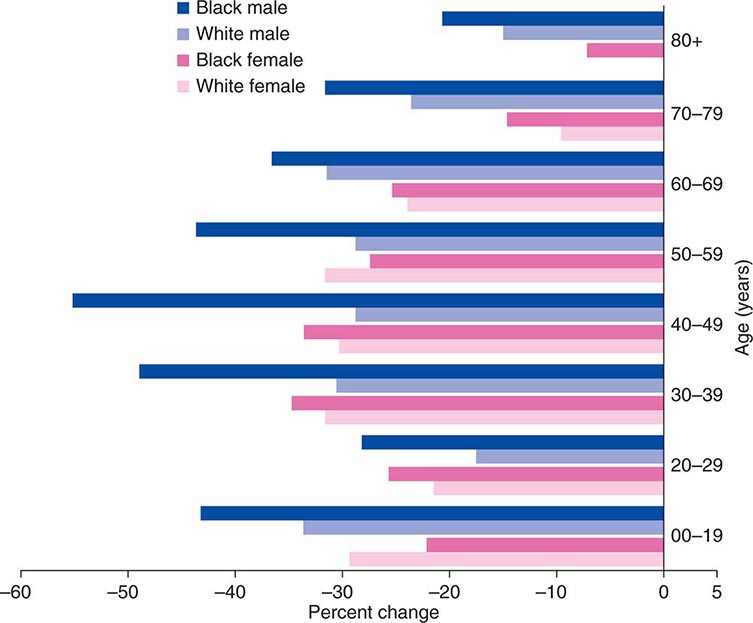
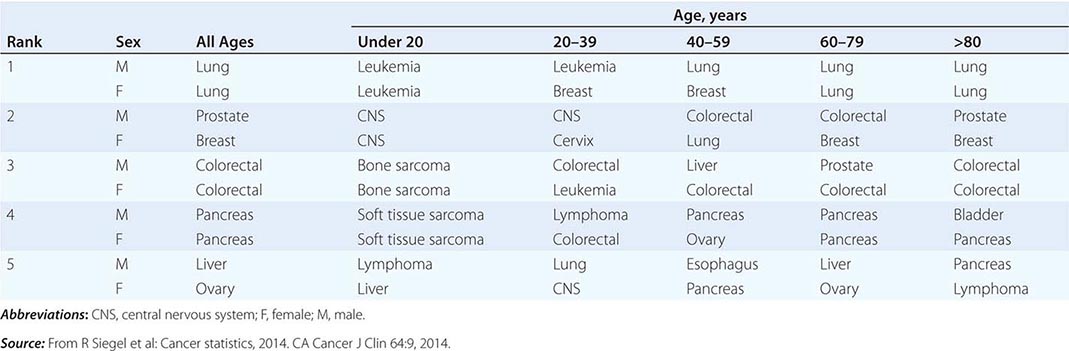
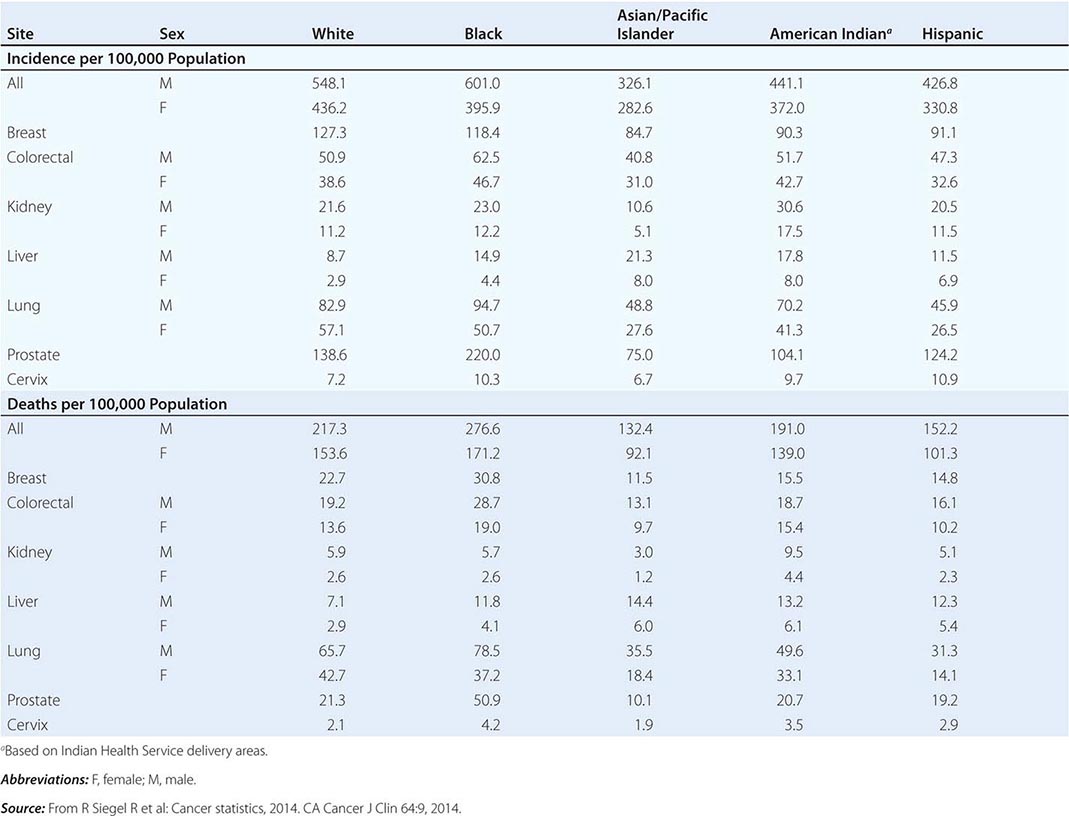
Cancer is the second leading cause of death behind heart disease. Deaths from heart disease have declined 45% in the United States since 1950 and continue to decline. Cancer has overtaken heart disease as the number one cause of death in persons younger than age 85 years. Incidence trends over time are shown in Fig. 99-1. After a 70-year period of increase, cancer deaths began to decline in 1990–1991 (Fig. 99-2). Between 1990 and 2010, cancer deaths decreased by 21% among men and 12.3% among women. The magnitude of the decline is illustrated in Fig. 99-3. The five leading causes of cancer deaths are shown for various populations in Table 99-2. The 5-year survival for white patients was 39% in 1960–1963 and 69% in 2003–2009. Cancers are more often deadly in blacks; the 5-year survival was 61% for the 2003–2009 interval; however, the racial differences are narrowing over time. Incidence and mortality vary among racial and ethnic groups (Table 99-3). The basis for these differences is unclear.
FIGURE 99-1 Incidence rates for particular types of cancer over the last 35 years in men (A) and women (B). (From R Siegel et al: CA Cancer J Clin 64:9, 2014.)
FIGURE 99-2 Eighty-year trend in cancer death rates for (A) women and (B) men by site in the United States, 1930–2010. Rates are per 100,000 age-adjusted to the 2000 U.S. standard population. All sites combined (A), individual sites in men (B) and individual sites in women (C) are shown. (From R Siegel et al: CA Cancer J Clin 64:9, 2014.)
FIGURE 99-3 The decline in death rates from cancer is shown for different age ranges by sex and race for the 20-year period between 1991 and 2010 expressed as a percentage of the 1991 rate. (From R Siegel et al: CA Cancer J Clin 64:9, 2014.)
THE FIVE LEADING PRIMARY TUMOR SITES FOR PATIENTS DYING OF CANCER BASED ON AGE AND SEX IN 2010 |
CANCER INCIDENCE AND MORTALITY IN RACIAL AND ETHNIC GROUPS, UNITED STATES, 2006–2010 |
CANCER AROUND THE WORLD
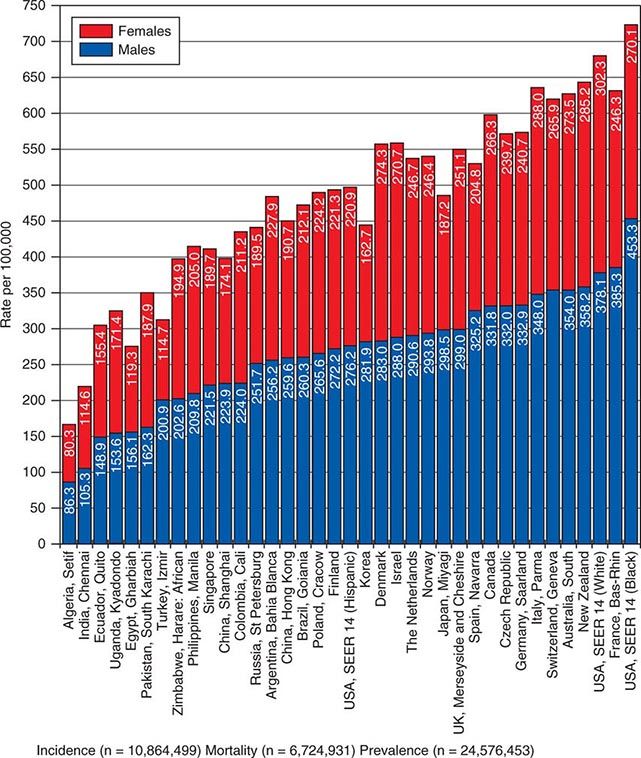
![]() In 2008, 12.7 million new cancer cases and 7.6 million cancer deaths were estimated worldwide, according to estimates of GLOBOCAN 2008, developed by the International Agency for Research on Cancer (IARC). When broken down by region of the world, ~45% of cases were in Asia, 26% in Europe, 14.5% in North America, 7.1% in Central/South America, 6% in Africa, and 1% in Australia/New Zealand (Fig. 99-4). Lung cancer is the most common cancer and the most common cause of cancer death in the world. Its incidence is highly variable, affecting only 2 per 100,000 African women but as many as 61 per 100,000 North American men. Breast cancer is the second most common cancer worldwide; however, it ranks fifth as a cause of death behind lung, stomach, liver, and colorectal cancer. Among the eight most common forms of cancer, lung (2-fold), breast (3-fold), prostate (2.5-fold), and colorectal (3-fold) cancers are more common in more developed countries than in less developed countries. By contrast, liver (2-fold), cervical (2-fold), and esophageal (2- to 3-fold) cancers are more common in less developed countries. Stomach cancer incidence is similar in more and less developed countries but is much more common in Asia than North America or Africa. The most common cancers in Africa are cervical, breast, and liver cancers. It has been estimated that nine modifiable risk factors are responsible for more than one-third of cancers worldwide. These include smoking, alcohol consumption, obesity, physical inactivity, low fruit and vegetable consumption, unsafe sex, air pollution, indoor smoke from household fuels, and contaminated injections.
In 2008, 12.7 million new cancer cases and 7.6 million cancer deaths were estimated worldwide, according to estimates of GLOBOCAN 2008, developed by the International Agency for Research on Cancer (IARC). When broken down by region of the world, ~45% of cases were in Asia, 26% in Europe, 14.5% in North America, 7.1% in Central/South America, 6% in Africa, and 1% in Australia/New Zealand (Fig. 99-4). Lung cancer is the most common cancer and the most common cause of cancer death in the world. Its incidence is highly variable, affecting only 2 per 100,000 African women but as many as 61 per 100,000 North American men. Breast cancer is the second most common cancer worldwide; however, it ranks fifth as a cause of death behind lung, stomach, liver, and colorectal cancer. Among the eight most common forms of cancer, lung (2-fold), breast (3-fold), prostate (2.5-fold), and colorectal (3-fold) cancers are more common in more developed countries than in less developed countries. By contrast, liver (2-fold), cervical (2-fold), and esophageal (2- to 3-fold) cancers are more common in less developed countries. Stomach cancer incidence is similar in more and less developed countries but is much more common in Asia than North America or Africa. The most common cancers in Africa are cervical, breast, and liver cancers. It has been estimated that nine modifiable risk factors are responsible for more than one-third of cancers worldwide. These include smoking, alcohol consumption, obesity, physical inactivity, low fruit and vegetable consumption, unsafe sex, air pollution, indoor smoke from household fuels, and contaminated injections.
FIGURE 99-4 Worldwide overall annual cancer incidence, mortality, and 5-year prevalence for the period of 1993–2001. (Adapted from A Jemal et al: Cancer Epidemiol Biomarkers Prev 19:1893, 2010.)
PATIENT MANAGEMENT
Important information is obtained from every portion of the routine history and physical examination. The duration of symptoms may reveal the chronicity of disease. The past medical history may alert the physician to the presence of underlying diseases that may affect the choice of therapy or the side effects of treatment. The social history may reveal occupational exposure to carcinogens or habits, such as smoking or alcohol consumption, that may influence the course of disease and its treatment. The family history may suggest an underlying familial cancer predisposition and point out the need to begin surveillance or other preventive therapy for unaffected siblings of the patient. The review of systems may suggest early symptoms of metastatic disease or a paraneoplastic syndrome.
DIAGNOSIS
The diagnosis of cancer relies most heavily on invasive tissue biopsy and should never be made without obtaining tissue; no noninvasive diagnostic test is sufficient to define a disease process as cancer. Although in rare clinical settings (e.g., thyroid nodules), fine-needle aspiration is an acceptable diagnostic procedure, the diagnosis generally depends on obtaining adequate tissue to permit careful evaluation of the histology of the tumor, its grade, and its invasiveness and to yield further molecular diagnostic information, such as the expression of cell-surface markers or intracellular proteins that typify a particular cancer, or the presence of a molecular marker, such as the t(8;14) translocation of Burkitt’s lymphoma. Increasing evidence links the expression of certain genes with the prognosis and response to therapy (Chaps. 101e and 102e).
Occasionally a patient will present with a metastatic disease process that is defined as cancer on biopsy but has no apparent primary site of disease. Efforts should be made to define the primary site based on age, sex, sites of involvement, histology and tumor markers, and personal and family history. Particular attention should be focused on ruling out the most treatable causes (Chap. 120e).
Once the diagnosis of cancer is made, the management of the patient is best undertaken as a multidisciplinary collaboration among the primary care physician, medical oncologists, surgical oncologists, radiation oncologists, oncology nurse specialists, pharmacists, social workers, rehabilitation medicine specialists, and a number of other consulting professionals working closely with each other and with the patient and family.
DEFINING THE EXTENT OF DISEASE AND THE PROGNOSIS
The first priority in patient management after the diagnosis of cancer is established and shared with the patient is to determine the extent of disease. The curability of a tumor usually is inversely proportional to the tumor burden. Ideally, the tumor will be diagnosed before symptoms develop or as a consequence of screening efforts (Chap. 100). A very high proportion of such patients can be cured. However, most patients with cancer present with symptoms related to the cancer, caused either by mass effects of the tumor or by alterations associated with the production of cytokines or hormones by the tumor.
For most cancers, the extent of disease is evaluated by a variety of noninvasive and invasive diagnostic tests and procedures. This process is called staging. There are two types. Clinical staging is based on physical examination, radiographs, isotopic scans, computed tomography (CT) scans, and other imaging procedures; pathologic staging takes into account information obtained during a surgical procedure, which might include intraoperative palpation, resection of regional lymph nodes and/or tissue adjacent to the tumor, and inspection and biopsy of organs commonly involved in disease spread. Pathologic staging includes histologic examination of all tissues removed during the surgical procedure. Surgical procedures performed may include a simple lymph node biopsy or more extensive procedures such as thoracotomy, mediastinoscopy, or laparotomy. Surgical staging may occur in a separate procedure or may be done at the time of definitive surgical resection of the primary tumor.
Knowledge of the predilection of particular tumors for spreading to adjacent or distant organs helps direct the staging evaluation.
Information obtained from staging is used to define the extent of disease as localized, as exhibiting spread outside of the organ of origin to regional but not distant sites, or as metastatic to distant sites. The most widely used system of staging is the TNM (tumor, node, metastasis) system codified by the International Union Against Cancer and the American Joint Committee on Cancer. The TNM classification is an anatomically based system that categorizes the tumor on the basis of the size of the primary tumor lesion (T1–4, where a higher number indicates a tumor of larger size), the presence of nodal involvement (usually N0 and N1 for the absence and presence, respectively, of involved nodes, although some tumors have more elaborate systems of nodal grading), and the presence of metastatic disease (M0 and M1 for the absence and presence, respectively, of metastases). The various permutations of T, N, and M scores (sometimes including tumor histologic grade [G]) are then broken into stages, usually designated by the roman numerals I through IV. Tumor burden increases and curability decreases with increasing stage. Other anatomic staging systems are used for some tumors, e.g., the Dukes classification for colorectal cancers, the International Federation of Gynecologists and Obstetricians classification for gynecologic cancers, and the Ann Arbor classification for Hodgkin’s disease.
Certain tumors cannot be grouped on the basis of anatomic considerations. For example, hematopoietic tumors such as leukemia, myeloma, and lymphoma are often disseminated at presentation and do not spread like solid tumors. For these tumors, other prognostic factors have been identified (Chaps. 132–136).
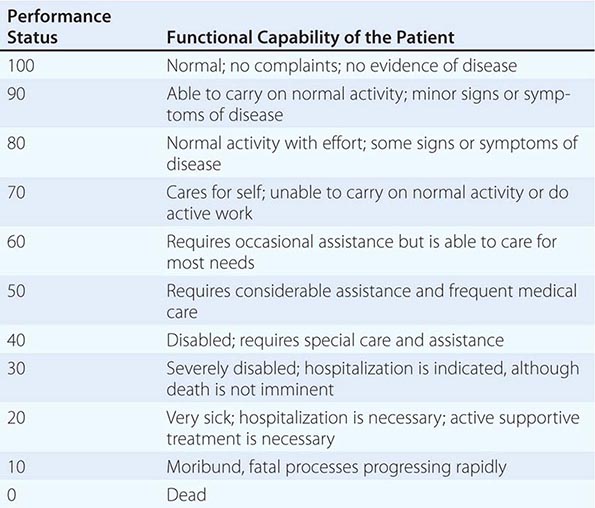
In addition to tumor burden, a second major determinant of treatment outcome is the physiologic reserve of the patient. Patients who are bedridden before developing cancer are likely to fare worse, stage for stage, than fully active patients. Physiologic reserve is a determinant of how a patient is likely to cope with the physiologic stresses imposed by the cancer and its treatment. This factor is difficult to assess directly. Instead, surrogate markers for physiologic reserve are used, such as the patient’s age or Karnofsky performance status (Table 99-4) or Eastern Cooperative Oncology Group (ECOG) performance status (Table 99-5). Older patients and those with a Karnofsky performance status <70 or ECOG performance status ≥3 have a poor prognosis unless the poor performance is a reversible consequence of the tumor.
KARNOFSKY PERFORMANCE INDEX |
THE EASTERN COOPERATIVE ONCOLOGY GROUP (ECOG) PERFORMANCE SCALE |
Source: From MM Oken et al: Am J Clin Oncol 5:649, 1982.
Increasingly, biologic features of the tumor are being related to prognosis. The expression of particular oncogenes, drug-resistance genes, apoptosis-related genes, and genes involved in metastasis is being found to influence response to therapy and prognosis. The presence of selected cytogenetic abnormalities may influence survival. Tumors with higher growth fractions, as assessed by expression of proliferation-related markers such as proliferating cell nuclear antigen, behave more aggressively than tumors with lower growth fractions. Information obtained from studying the tumor itself will increasingly be used to influence treatment decisions. Host genes involved in drug metabolism can influence the safety and efficacy of particular treatments.
Enormous heterogeneity has been noted by studying tumors; we have learned that morphology is not capable of discerning certain distinct subsets of patients whose tumors have different sets of abnormalities. Tumors that look the same by light microscopy can be very different. Similarly, tumors that look quite different from one another histologically can share genetic lesions that predict responses to treatments. Furthermore, tumor cells vary enormously within a single patient even though the cells share a common origin.
MAKING A TREATMENT PLAN
From information on the extent of disease and the prognosis and in conjunction with the patient’s wishes, it is determined whether the treatment approach should be curative or palliative in intent. Cooperation among the various professionals involved in cancer treatment is of the utmost importance in treatment planning. For some cancers, chemotherapy or chemotherapy plus radiation therapy delivered before the use of definitive surgical treatment (so-called neoadjuvant therapy) may improve the outcome, as seems to be the case for locally advanced breast cancer and head and neck cancers. In certain settings in which combined-modality therapy is intended, coordination among the medical oncologist, radiation oncologist, and surgeon is crucial to achieving optimal results. Sometimes the chemotherapy and radiation therapy need to be delivered sequentially, and other times concurrently. Surgical procedures may precede or follow other treatment approaches. It is best for the treatment plan either to follow a standard protocol precisely or else to be part of an ongoing clinical research protocol evaluating new treatments. Ad hoc modifications of standard protocols are likely to compromise treatment results.
The choice of treatment approaches was formerly dominated by the local culture in both the university and the practice settings. However, it is now possible to gain access electronically to standard treatment protocols and to every approved clinical research study in North America through a personal computer interface with the Internet.1
The skilled physician also has much to offer the patient for whom curative therapy is no longer an option. Often a combination of guilt and frustration over the inability to cure the patient and the pressure of a busy schedule greatly limit the time a physician spends with a patient who is receiving only palliative care. Resist these forces. In addition to the medicines administered to alleviate symptoms (see below), it is important to remember the comfort that is provided by holding the patient’s hand, continuing regular examinations, and taking time to talk.
MANAGEMENT OF DISEASE AND TREATMENT COMPLICATIONS
Because cancer therapies are toxic (Chap. 103e), patient management involves addressing complications of both the disease and its treatment as well as the complex psychosocial problems associated with cancer. In the short term during a course of curative therapy, the patient’s functional status may decline. Treatment-induced toxicity is less acceptable if the goal of therapy is palliation. The most common side effects of treatment are nausea and vomiting (see below), febrile neutropenia (Chap. 104), and myelosuppression (Chap. 103e). Tools are now available to minimize the acute toxicity of cancer treatment.
New symptoms developing in the course of cancer treatment should always be assumed to be reversible until proven otherwise. The fatalistic attribution of anorexia, weight loss, and jaundice to recurrent or progressive tumor could result in a patient dying from a reversible intercurrent cholecystitis. Intestinal obstruction may be due to reversible adhesions rather than progressive tumor. Systemic infections, sometimes with unusual pathogens, may be a consequence of the immunosuppression associated with cancer therapy. Some drugs used to treat cancer or its complications (e.g., nausea) may produce central nervous system symptoms that look like metastatic disease or may mimic paraneoplastic syndromes such as the syndrome of inappropriate antidiuretic hormone. A definitive diagnosis should be pursued and may even require a repeat biopsy.
A critical component of cancer management is assessing the response to treatment. In addition to a careful physical examination in which all sites of disease are physically measured and recorded in a flow chart by date, response assessment usually requires periodic repeating of imaging tests that were abnormal at the time of staging. If imaging tests have become normal, repeat biopsy of previously involved tissue is performed to document complete response by pathologic criteria. Biopsies are not usually required if there is macroscopic residual disease. A complete response is defined as disappearance of all evidence of disease, and a partial response as >50% reduction in the sum of the products of the perpendicular diameters of all measurable lesions. The determination of partial response may also be based on a 30% decrease in the sums of the longest diameters of lesions (Response Evaluation Criteria in Solid Tumors [RECIST]). Progressive disease is defined as the appearance of any new lesion or an increase of >25% in the sum of the products of the perpendicular diameters of all measurable lesions (or an increase of 20% in the sums of the longest diameters by RECIST). Tumor shrinkage or growth that does not meet any of these criteria is considered stable disease. Some sites of involvement (e.g., bone) or patterns of involvement (e.g., lymphangitic lung or diffuse pulmonary infiltrates) are considered unmeasurable. No response is complete without biopsy documentation of their resolution, but partial responses may exclude their assessment unless clear objective progression has occurred.
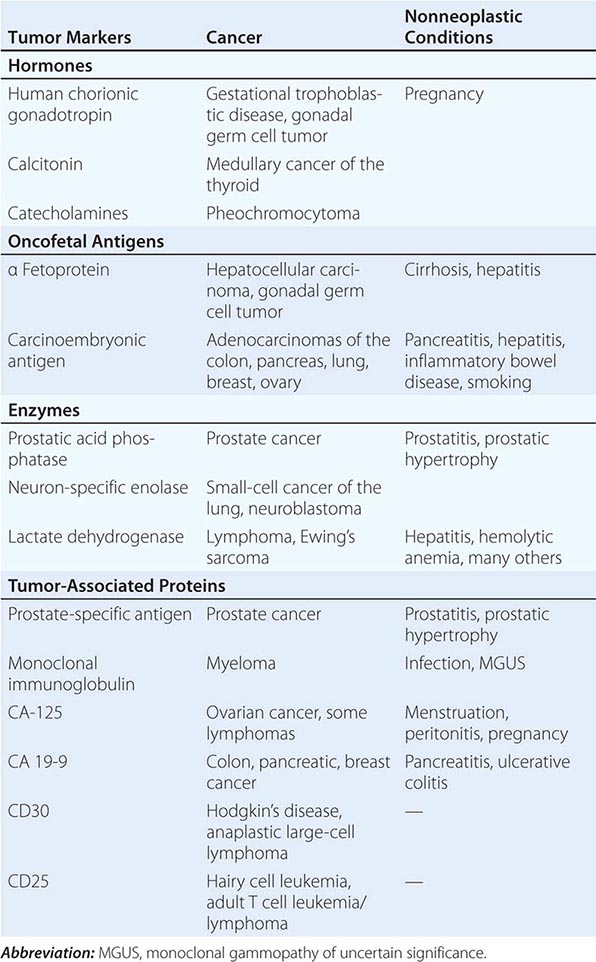
Tumor markers may be useful in patient management in certain tumors. Response to therapy may be difficult to gauge with certainty. However, some tumors produce or elicit the production of markers that can be measured in the serum or urine, and in a particular patient, rising and falling levels of the marker are usually associated with increasing or decreasing tumor burden, respectively. Some clinically useful tumor markers are shown in Table 99-6. Tumor markers are not in themselves specific enough to permit a diagnosis of malignancy to be made, but once a malignancy has been diagnosed and shown to be associated with elevated levels of a tumor marker, the marker can be used to assess response to treatment.
TUMOR MARKERS |
The recognition and treatment of depression are important components of management. The incidence of depression in cancer patients is ~25% overall and may be greater in patients with greater debility. This diagnosis is likely in a patient with a depressed mood (dysphoria) and/or a loss of interest in pleasure (anhedonia) for at least 2 weeks. In addition, three or more of the following symptoms are usually present: appetite change, sleep problems, psychomotor retardation or agitation, fatigue, feelings of guilt or worthlessness, inability to concentrate, and suicidal ideation. Patients with these symptoms should receive therapy. Medical therapy with a serotonin reuptake inhibitor such as fluoxetine (10–20 mg/d), sertraline (50–150 mg/d), or paroxetine (10–20 mg/d) or a tricyclic antidepressant such as amitriptyline (50–100 mg/d) or desipramine (75–150 mg/d) should be tried, allowing 4–6 weeks for response. Effective therapy should be continued at least 6 months after resolution of symptoms. If therapy is unsuccessful, other classes of antidepressants may be used. In addition to medication, psychosocial interventions such as support groups, psychotherapy, and guided imagery may be of benefit.
Many patients opt for unproven or unsound approaches to treatment when it appears that conventional medicine is unlikely to be curative. Those seeking such alternatives are often well educated and may be early in the course of their disease. Unsound approaches are usually hawked on the basis of unsubstantiated anecdotes and not only cannot help the patient but may be harmful. Physicians should strive to keep communications open and nonjudgmental, so that patients are more likely to discuss with the physician what they are actually doing. The appearance of unexpected toxicity may be an indication that a supplemental therapy is being taken.2
LONG-TERM FOLLOW-UP/LATE COMPLICATIONS
At the completion of treatment, sites originally involved with tumor are reassessed, usually by radiography or imaging techniques, and any persistent abnormality is biopsied. If disease persists, the multidisciplinary team discusses a new salvage treatment plan. If the patient has been rendered disease-free by the original treatment, the patient is followed regularly for disease recurrence. The optimal guidelines for follow-up care are not known. For many years, a routine practice has been to follow the patient monthly for 6–12 months, then every other month for a year, every 3 months for a year, every 4 months for a year, every 6 months for a year, and then annually. At each visit, a battery of laboratory and radiographic and imaging tests were obtained on the assumption that it is best to detect recurrent disease before it becomes symptomatic. However, where follow-up procedures have been examined, this assumption has been found to be untrue. Studies of breast cancer, melanoma, lung cancer, colon cancer, and lymphoma have all failed to support the notion that asymptomatic relapses are more readily cured by salvage therapy than symptomatic relapses. In view of the enormous cost of a full battery of diagnostic tests and their manifest lack of impact on survival, new guidelines are emerging for less frequent follow-up visits, during which the history and physical examination are the major investigations performed.
As time passes, the likelihood of recurrence of the primary cancer diminishes. For many types of cancer, survival for 5 years without recurrence is tantamount to cure. However, important medical problems can occur in patients treated for cancer and must be examined (Chap. 125). Some problems emerge as a consequence of the disease and some as a consequence of the treatment. An understanding of these disease- and treatment-related problems may help in their detection and management.
Despite these concerns, most patients who are cured of cancer return to normal lives.
SUPPORTIVE CARE
In many ways, the success of cancer therapy depends on the success of the supportive care. Failure to control the symptoms of cancer and its treatment may lead patients to abandon curative therapy. Of equal importance, supportive care is a major determinant of quality of life. Even when life cannot be prolonged, the physician must strive to preserve its quality. Quality-of-life measurements have become common endpoints of clinical research studies. Furthermore, palliative care has been shown to be cost-effective when approached in an organized fashion. A credo for oncology could be to cure sometimes, to extend life often, and to comfort always.
Pain Pain occurs with variable frequency in the cancer patient: 25–50% of patients present with pain at diagnosis, 33% have pain associated with treatment, and 75% have pain with progressive disease. The pain may have several causes. In ~70% of cases, pain is caused by the tumor itself—by invasion of bone, nerves, blood vessels, or mucous membranes or obstruction of a hollow viscus or duct. In ~20% of cases, pain is related to a surgical or invasive medical procedure, to radiation injury (mucositis, enteritis, or plexus or spinal cord injury), or to chemotherapy injury (mucositis, peripheral neuropathy, phlebitis, steroid-induced aseptic necrosis of the femoral head). In 10% of cases, pain is unrelated to cancer or its treatment.
Assessment of pain requires the methodical investigation of the history of the pain, its location, character, temporal features, provocative and palliative factors, and intensity (Chap. 18); a review of the oncologic history and past medical history as well as personal and social history; and a thorough physical examination. The patient should be given a 10-division visual analogue scale on which to indicate the severity of the pain. The clinical condition is often dynamic, making it necessary to reassess the patient frequently. Pain therapy should not be withheld while the cause of pain is being sought.
A variety of tools are available with which to address cancer pain. About 85% of patients will have pain relief from pharmacologic intervention. However, other modalities, including antitumor therapy (such as surgical relief of obstruction, radiation therapy, and strontium-89 or samarium-153 treatment for bone pain), neurostimulatory techniques, regional analgesia, or neuroablative procedures, are effective in an additional 12% or so. Thus, very few patients will have inadequate pain relief if appropriate measures are taken. A specific approach to pain relief is detailed in Chap. 10.
Nausea Emesis in the cancer patient is usually caused by chemotherapy (Chap. 103e). Its severity can be predicted from the drugs used to treat the cancer. Three forms of emesis are recognized on the basis of their timing with regard to the noxious insult. Acute emesis, the most common variety, occurs within 24 h of treatment. Delayed emesis occurs 1–7 days after treatment; it is rare, but, when present, usually follows cisplatin administration. Anticipatory emesis occurs before administration of chemotherapy and represents a conditioned response to visual and olfactory stimuli previously associated with chemotherapy delivery.
Acute emesis is the best understood form. Stimuli that activate signals in the chemoreceptor trigger zone in the medulla, the cerebral cortex, and peripherally in the intestinal tract lead to stimulation of the vomiting center in the medulla, the motor center responsible for coordinating the secretory and muscle contraction activity that leads to emesis. Diverse receptor types participate in the process, including dopamine, serotonin, histamine, opioid, and acetylcholine receptors. The serotonin receptor antagonists ondansetron and granisetron are the most effective drugs against highly emetogenic agents, but they are expensive.
As with the analgesia ladder, emesis therapy should be tailored to the situation. For mildly and moderately emetogenic agents, prochlorperazine, 5–10 mg PO or 25 mg PR, is effective. Its efficacy may be enhanced by administering the drug before the chemotherapy is delivered. Dexamethasone, 10–20 mg IV, is also effective and may enhance the efficacy of prochlorperazine. For highly emetogenic agents such as cisplatin, mechlorethamine, dacarbazine, and streptozocin, combinations of agents work best and administration should begin 6–24 h before treatment. Ondansetron, 8 mg PO every 6 h the day before therapy and IV on the day of therapy, plus dexamethasone, 20 mg IV before treatment, is an effective regimen. Addition of oral aprepitant (a substance P/neurokinin 1 receptor antagonist) to this regimen (125 mg on day 1, 80 mg on days 2 and 3) further decreases the risk of both acute and delayed vomiting. Like pain, emesis is easier to prevent than to alleviate.
Delayed emesis may be related to bowel inflammation from the therapy and can be controlled with oral dexamethasone and oral metoclopramide, a dopamine receptor antagonist that also blocks serotonin receptors at high dosages. The best strategy for preventing anticipatory emesis is to control emesis in the early cycles of therapy to prevent the conditioning from taking place. If this is unsuccessful, prophylactic antiemetics the day before treatment may help. Experimental studies are evaluating behavior modification.
Effusions Fluid may accumulate abnormally in the pleural cavity, pericardium, or peritoneum. Asymptomatic malignant effusions may not require treatment. Symptomatic effusions occurring in tumors responsive to systemic therapy usually do not require local treatment but respond to the treatment for the underlying tumor. Symptomatic effusions occurring in tumors unresponsive to systemic therapy may require local treatment in patients with a life expectancy of at least 6 months.
Pleural effusions due to tumors may or may not contain malignant cells. Lung cancer, breast cancer, and lymphomas account for ~75% of malignant pleural effusions. Their exudative nature is usually gauged by an effusion/serum protein ratio of ≥0.5 or an effusion/serum lactate dehydrogenase ratio of ≥0.6. When the condition is symptomatic, thoracentesis is usually performed first. In most cases, symptomatic improvement occurs for <1 month. Chest tube drainage is required if symptoms recur within 2 weeks. Fluid is aspirated until the flow rate is <100 mL in 24 h. Then either 60 units of bleomycin or 1 g of doxycycline is infused into the chest tube in 50 mL of 5% dextrose in water; the tube is clamped; the patient is rotated on four sides, spending 15 min in each position; and, after 1–2 h, the tube is again attached to suction for another 24 h. The tube is then disconnected from suction and allowed to drain by gravity. If <100 mL drains over the next 24 h, the chest tube is pulled, and a radiograph is taken 24 h later. If the chest tube continues to drain fluid at an unacceptably high rate, sclerosis can be repeated. Bleomycin may be somewhat more effective than doxycycline but is very expensive. Doxycycline is usually the drug of first choice. If neither doxycycline nor bleomycin is effective, talc can be used.
Symptomatic pericardial effusions are usually treated by creating a pericardial window or by stripping the pericardium. If the patient’s condition does not permit a surgical procedure, sclerosis can be attempted with doxycycline and/or bleomycin.
Malignant ascites is usually treated with repeated paracentesis of small volumes of fluid. If the underlying malignancy is unresponsive to systemic therapy, peritoneovenous shunts may be inserted. Despite the fear of disseminating tumor cells into the circulation, widespread metastases are an unusual complication. The major complications are occlusion, leakage, and fluid overload. Patients with severe liver disease may develop disseminated intravascular coagulation.
Nutrition Cancer and its treatment may lead to a decrease in nutrient intake of sufficient magnitude to cause weight loss and alteration of intermediary metabolism. The prevalence of this problem is difficult to estimate because of variations in the definition of cancer cachexia, but most patients with advanced cancer experience weight loss and decreased appetite. A variety of both tumor-derived factors (e.g., bombesin, adrenocorticotropic hormone) and host-derived factors (e.g., tumor necrosis factor, interleukins 1 and 6, growth hormone) contribute to the altered metabolism, and a vicious cycle is established in which protein catabolism, glucose intolerance, and lipolysis cannot be reversed by the provision of calories.
It remains controversial how to assess nutritional status and when and how to intervene. Efforts to make the assessment objective have included the use of a prognostic nutritional index based on albumin levels, triceps skinfold thickness, transferrin levels, and delayed-type hypersensitivity skin testing. However, a simpler approach has been to define the threshold for nutritional intervention as <10% unexplained body weight loss, serum transferrin level <1500 mg/L (150 mg/dL), and serum albumin <34 g/L (3.4 g/dL).
The decision is important, because it appears that cancer therapy is substantially more toxic and less effective in the face of malnutrition. Nevertheless, it remains unclear whether nutritional intervention can alter the natural history. Unless some pathology is affecting the absorptive function of the gastrointestinal tract, enteral nutrition provided orally or by tube feeding is preferred over parenteral supplementation. However, the risks associated with the tube may outweigh the benefits. Megestrol acetate, a progestational agent, has been advocated as a pharmacologic intervention to improve nutritional status. Research in this area may provide more tools in the future as cytokine-mediated mechanisms are further elucidated.
Psychosocial Support The psychosocial needs of patients vary with their situation. Patients undergoing treatment experience fear, anxiety, and depression. Self-image is often seriously compromised by deforming surgery and loss of hair. Women who receive cosmetic advice that enables them to look better also feel better. Loss of control over how one spends time can contribute to the sense of vulnerability. Juggling the demands of work and family with the demands of treatment may create enormous stresses. Sexual dysfunction is highly prevalent and needs to be discussed openly with the patient. An empathetic health care team is sensitive to the individual patient’s needs and permits negotiation where such flexibility will not adversely affect the course of treatment.
Cancer survivors have other sets of difficulties. Patients may have fears associated with the termination of a treatment they associate with their continued survival. Adjustments are required to physical losses and handicaps, real and perceived. Patients may be preoccupied with minor physical problems. They perceive a decline in their job mobility and view themselves as less desirable workers. They may be victims of job and/or insurance discrimination. Patients may experience difficulty reentering their normal past life. They may feel guilty for having survived and may carry a sense of vulnerability to colds and other illnesses. Perhaps the most pervasive and threatening concern is the ever-present fear of relapse (the Damocles syndrome).
Patients in whom therapy has been unsuccessful have other problems related to the end of life.
Death and Dying The most common causes of death in patients with cancer are infection (leading to circulatory failure), respiratory failure, hepatic failure, and renal failure. Intestinal blockage may lead to inanition and starvation. Central nervous system disease may lead to seizures, coma, and central hypoventilation. About 70% of patients develop dyspnea preterminally. However, many months usually pass between the diagnosis of cancer and the occurrence of these complications, and during this period, the patient is severely affected by the possibility of death. The path of unsuccessful cancer treatment usually occurs in three phases. First, there is optimism at the hope of cure; when the tumor recurs, there is the acknowledgment of an incurable disease, and the goal of palliative therapy is embraced in the hope of being able to live with disease; finally, at the disclosure of imminent death, another adjustment in outlook takes place. The patient imagines the worst in preparation for the end of life and may go through stages of adjustment to the diagnosis. These stages include denial, isolation, anger, bargaining, depression, acceptance, and hope. Of course, patients do not all progress through all the stages or proceed through them in the same order or at the same rate. Nevertheless, developing an understanding of how the patient has been affected by the diagnosis and is coping with it is an important goal of patient management.
It is best to speak frankly with the patient and the family regarding the likely course of disease. These discussions can be difficult for the physician as well as for the patient and family. The critical features of the interaction are to reassure the patient and family that everything that can be done to provide comfort will be done. They will not be abandoned. Many patients prefer to be cared for in their homes or in a hospice setting rather than a hospital. The American College of Physicians has published a book called Home Care Guide for Cancer: How to Care for Family and Friends at Home that teaches an approach to successful problem-solving in home care. With appropriate planning, it should be possible to provide the patient with the necessary medical care as well as the psychological and spiritual support that will prevent the isolation and depersonalization that can attend in-hospital death.
The care of dying patients may take a toll on the physician. A “burnout” syndrome has been described that is characterized by fatigue, disengagement from patients and colleagues, and a loss of self-fulfillment. Efforts at stress reduction, maintenance of a balanced life, and setting realistic goals may combat this disorder.
End-of-Life Decisions Unfortunately, a smooth transition in treatment goals from curative to palliative may not be possible in all cases because of the occurrence of serious treatment-related complications or rapid disease progression. Vigorous and invasive medical support for a reversible disease or treatment complication is assumed to be justified. However, if the reversibility of the condition is in doubt, the patient’s wishes determine the level of medical care. These wishes should be elicited before the terminal phase of illness and reviewed periodically. Information about advance directives can be obtained from the American Association of Retired Persons, 601 E Street, NW, Washington, DC 20049, 202-434-2277, or Choice in Dying, 250 West 57th Street, New York, NY 10107, 212-366-5540. Some states allow physicians to assist patients who choose to end their lives. This subject is challenging from an ethical and a medical point of view. Discussions of end-of-life decisions should be candid and involve clear informed consent, waiting periods, second opinions, and documentation. A full discussion of end-of-life management is in Chap. 10.
_____________________________
1The National Cancer Institute maintains a database called PDQ (Physician Data Query) that is accessible on the Internet under the name CancerNet at www.cancer.gov/cancertopics/pdq/cancerdatabase. Information can be obtained through a facsimile machine using CancerFax by dialing 301-402-5874. Patient information is also provided by the National Cancer Institute in at least three formats: on the Internet via CancerNet at www.cancer.gov, through the CancerFax number listed above, or by calling 1-800-4-CANCER. The quality control for the information provided through these services is rigorous.
2Information about unsound methods may be obtained from the National Council Against Health Fraud, Box 1276, Loma Linda, CA 92354, or from the Center for Medical Consumers and Health Care Information, 237 Thompson Street, New York, NY 10012.
100 | Prevention and Early Detection of Cancer |
Improved understanding of carcinogenesis has allowed cancer prevention and early detection (also known as cancer control) to expand beyond the identification and avoidance of carcinogens. Specific interventions to prevent cancer in those at risk, and effective screening for early detection of cancer, are the goals.
Carcinogenesis is not an event but a process, a continuum of discrete tissue and cellular changes over time resulting in aberrant physiologic processes. Prevention concerns the identification and manipulation of the biologic, environmental, social, and genetic factors in the causal pathway of cancer.
EDUCATION AND HEALTHFUL HABITS
Public education on the avoidance of identified risk factors for cancer and encouraging healthy habits contributes to cancer prevention and control. The clinician is a powerful messenger in this process. The patient-provider encounter provides an opportunity to teach patients about the hazards of smoking, the features of a healthy lifestyle, use of proven cancer screening methods, and avoidance of excessive sun exposure.
SMOKING CESSATION
Tobacco smoking is a strong, modifiable risk factor for cardiovascular disease, pulmonary disease, and cancer. Smokers have an approximately 1 in 3 lifetime risk of dying prematurely from a tobacco-related cancer, cardiovascular, or pulmonary disease. Tobacco use causes more deaths from cardiovascular disease than from cancer. Lung cancer and cancers of the larynx, oropharynx, esophagus, kidney, bladder, pancreas, and stomach are all tobacco-related.
The number of cigarettes smoked per day and the level of inhalation of cigarette smoke are correlated with risk of lung cancer mortality. Light- and low-tar cigarettes are not safer, because smokers tend to inhale them more frequently and deeply.
Those who stop smoking have a 30–50% lower 10-year lung cancer mortality rate compared to those who continue smoking, despite the fact that some carcinogen-induced gene mutations persist for years after smoking cessation. Smoking cessation and avoidance would save more lives than any other public health activity.
The risk of tobacco smoke is not limited to the smoker. Environmental tobacco smoke, known as secondhand or passive smoke, causes lung cancer and other cardiopulmonary diseases in nonsmokers.
Tobacco use prevention is a pediatric issue. More than 80% of adult American smokers began smoking before the age of 18 years. Approximately 20% of Americans in grades 9 through 12 have smoked a cigarette in the past month. Counseling of adolescents and young adults is critical to prevent smoking. A clinician’s simple advice can be of benefit. Providers should query patients on tobacco use and offer smokers assistance in quitting.
Current approaches to smoking cessation recognize smoking as an addiction (Chap. 470). The smoker who is quitting goes through identifiable stages that include contemplation of quitting, an action phase in which the smoker quits, and a maintenance phase. Smokers who quit completely are more likely to be successful than those who gradually reduce the number of cigarettes smoked or change to lower-tar or lower-nicotine cigarettes. More than 90% of the Americans who have successfully quit smoking did so on their own, without participation in an organized cessation program, but cessation programs are helpful for some smokers. The Community Intervention Trial for Smoking Cessation (COMMIT) was a 4-year program showing that light smokers (<25 cigarettes per day) were more likely to benefit from simple cessation messages and cessation programs than those who did not receive an intervention. Quit rates were 30.6% in the intervention group and 27.5% in the control group. The COMMIT interventions were unsuccessful in heavy smokers (<25 cigarettes per day). Heavy smokers may need an intensive broad-based cessation program that includes counseling, behavioral strategies, and pharmacologic adjuncts, such as nicotine replacement (gum, patches, sprays, lozenges, and inhalers), bupropion, and/or varenicline.
The health risks of cigars are similar to those of cigarettes. Smoking one or two cigars daily doubles the risk for oral and esophageal cancers; smoking three or four cigars daily increases the risk of oral cancers more than eightfold and esophageal cancer fourfold. The risks of occasional use are unknown.
Smokeless tobacco also represents a substantial health risk. Chewing tobacco is a carcinogen linked to dental caries, gingivitis, oral leukoplakia, and oral cancer. The systemic effects of smokeless tobacco (including snuff) may increase risks for other cancers. Esophageal cancer is linked to carcinogens in tobacco dissolved in saliva and swallowed. The net effects of e-cigarettes on health are poorly studied. Whether they aid in smoking cessation or serve as a “gateway” for nonsmoking children to acquire a smoking habit is debated.
PHYSICAL ACTIVITY
Physical activity is associated with a decreased risk of colon and breast cancer. A variety of mechanisms have been proposed. However, such studies are prone to confounding factors such as recall bias, association of exercise with other health-related practices, and effects of preclinical cancers on exercise habits (reverse causality).
DIET MODIFICATION
International epidemiologic studies suggest that diets high in fat are associated with increased risk for cancers of the breast, colon, prostate, and endometrium. These cancers have their highest incidence and mortalities in Western cultures, where fat composes an average of one-third of the total calories consumed.
Despite correlations, dietary fat has not been proven to cause cancer. Case-control and cohort epidemiologic studies give conflicting results. In addition, diet is a highly complex exposure to many nutrients and chemicals. Low-fat diets are associated with many dietary changes beyond simple subtraction of fat. Other lifestyle changes are also associated with adherence to a low-fat diet.
In observational studies, dietary fiber is associated with a reduced risk of colonic polyps and invasive cancer of the colon. However, cancer-protective effects of increasing fiber and lowering dietary fat have not been proven in the context of a prospective clinical trial. The putative protective mechanisms are complex and speculative. Fiber binds oxidized bile acids and generates soluble fiber products, such as butyrate, that may have differentiating properties. Fiber does not increase bowel transit times. Two large prospective cohort studies of >100,000 health professionals showed no association between fruit and vegetable intake and risk of cancer.
The Polyp Prevention Trial randomly assigned 2000 elderly persons, who had polyps removed, to a low-fat, high-fiber diet versus routine diet for 4 years. No differences were noted in polyp formation.
The U.S. National Institutes of Health Women’s Health Initiative, launched in 1994, was a long-term clinical trial enrolling >100,000 women age 45–69 years. It placed women in 22 intervention groups. Participants received calcium/vitamin D supplementation; hormone replacement therapy; and counseling to increase exercise, eat a low-fat diet with increased consumption of fruits, vegetables, and fiber, and cease smoking. The study showed that although dietary fat intake was lower in the diet intervention group, invasive breast cancers were not reduced over an 8-year follow-up period compared to the control group. No reduction was seen in the incidence of colorectal cancer in the dietary intervention arm. The difference in dietary fat averaged ∼10% between the two groups. Evidence does not currently establish the anticarcinogenic value of vitamin, mineral, or nutritional supplements in amounts greater than those provided by a balanced diet.
ENERGY BALANCE
Risk of cancer appears to increase as body mass index increases beyond 25 kg/m2. Obesity is associated with increased risk for cancers of the colon, breast (female postmenopausal), endometrium, kidney (renal cell), and esophagus, although causality has not been established.
In observational studies, relative risks of colon cancer are increased in obesity by 1.5–2 for men and 1.2–1.5 for women. Obese postmenopausal women have a 30–50% increased relative risk of breast cancer. An unproven hypothesis for the association is that adipose tissue serves as a depot for aromatase that facilitates estrogen production.
SUN AVOIDANCE
Nonmelanoma skin cancers (basal cell and squamous cell) are induced by cumulative exposure to ultraviolet (UV) radiation. Intermittent acute sun exposure and sun damage have been linked to melanoma, but the evidence is inconsistent. Sunburns, especially in childhood and adolescence, may be associated with an increased risk of melanoma in adulthood. Reduction of sun exposure through use of protective clothing and changing patterns of outdoor activities can reduce skin cancer risk. Sunscreens decrease the risk of actinic keratoses, the precursor to squamous cell skin cancer, but melanoma risk may not be reduced. Sunscreens prevent burning, but they may encourage more prolonged exposure to the sun and may not filter out wavelengths of energy that cause melanoma.
Educational interventions to help individuals assess their risk of developing skin cancer have some impact. In particular, appearance-focused behavioral interventions in young women can decrease indoor tanning use and other UV exposures. Self-examination for skin pigment characteristics associated with skin cancer, such as freckling, may be useful in identifying people at high risk. Those who recognize themselves as being at risk tend to be more compliant with sun-avoidance recommendations. Risk factors for melanoma include a propensity to sunburn, a large number of benign melanocytic nevi, and atypical nevi.
CANCER CHEMOPREVENTION
Chemoprevention involves the use of specific natural or synthetic chemical agents to reverse, suppress, or prevent carcinogenesis before the development of invasive malignancy.
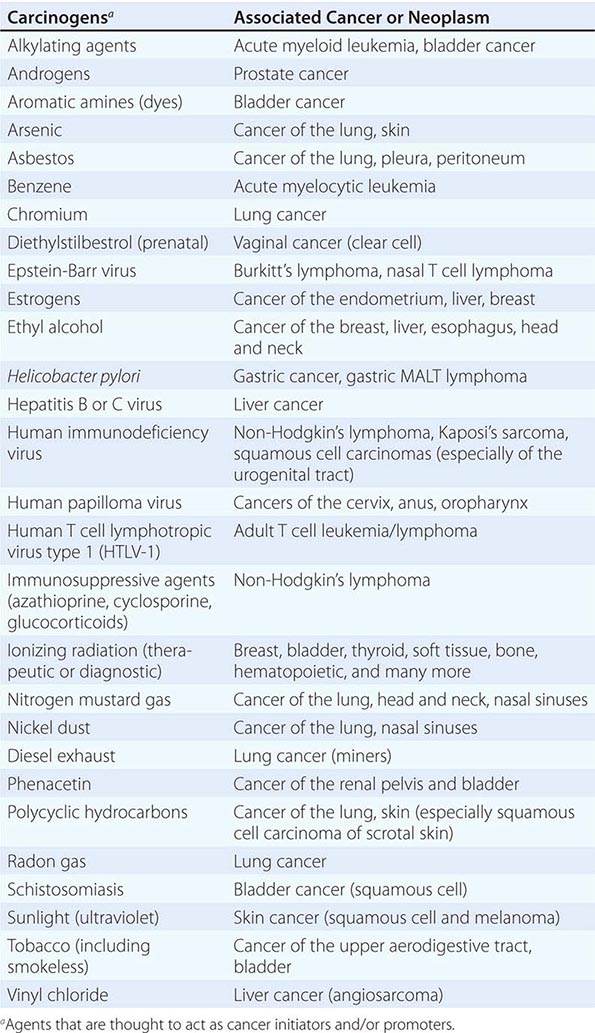
Cancer develops through an accumulation of tissue abnormalities associated with genetic and epigenetic changes, and growth regulatory pathways that are potential points of intervention to prevent cancer. The initial changes are termed initiation. The alteration can be inherited or acquired through the action of physical, infectious, or chemical carcinogens. Like most human diseases, cancer arises from an interaction between genetics and environmental exposures (Table 100-1). Influences that cause the initiated cell and its surrounding tissue microenvironment to progress through the carcinogenic process and change phenotypically are termed promoters. Promoters include hormones such as androgens, linked to prostate cancer, and estrogen, linked to breast and endometrial cancer. The distinction between an initiator and promoter is indistinct; some components of cigarette smoke are “complete carcinogens,” acting as both initiators and promoters. Cancer can be prevented or controlled through interference with the factors that cause cancer initiation, promotion, or progression. Compounds of interest in chemoprevention often have antimutagenic, hormone modulation, anti-inflammatory, antiproliferative, or proapoptotic activity (or a combination).
SUSPECTED CARCINOGENS |
CHEMOPREVENTION OF CANCERS OF THE UPPER AERODIGESTIVE TRACT
Smoking causes diffuse epithelial injury in the oral cavity, neck, esophagus, and lung. Patients cured of squamous cell cancers of the lung, esophagus, oral cavity, and neck are at risk (as high as 5% per year) of developing second cancers of the upper aerodigestive tract. Cessation of cigarette smoking does not markedly decrease the cured cancer patient’s risk of second malignancy, even though it does lower the cancer risk in those who have never developed a malignancy. Smoking cessation may halt the early stages of the carcinogenic process (such as metaplasia), but it may have no effect on late stages of carcinogenesis. This “field carcinogenesis” hypothesis for upper aerodigestive tract cancer has made “cured” patients an important population for chemoprevention of second malignancies.
Oral human papilloma virus (HPV) infection, particularly HPV-16, increases the risk for cancers of the oropharynx. This association exists even in the absence of other risk factors such as smoking or alcohol use (although the magnitude of increased risk appears greater than additive when HPV infection and smoking are both present). Oral HPV infection is believed to be largely sexually acquired. Although no direct evidence currently exists to confirm the hypothesis, the introduction of the HPV vaccine may eventually reduce oropharyngeal cancer rates.
Oral leukoplakia, a premalignant lesion commonly found in smokers, has been used as an intermediate marker of chemopreventive activity in smaller shorter-duration, randomized, placebo-controlled trials. Response was associated with upregulation of retinoic acid receptor-β (RAR-β). Therapy with high, relatively toxic doses of isotretinoin (13-cis-retinoic acid) causes regression of oral leukoplakia. However, the lesions recur when the therapy is withdrawn, suggesting the need for long-term administration. More tolerable doses of isotretinoin have not shown benefit in the prevention of head and neck cancer. Isotretinoin also failed to prevent second malignancies in patients cured of early-stage non-small cell lung cancer; mortality rates were actually increased in current smokers.
Several large-scale trials have assessed agents in the chemoprevention of lung cancer in patients at high risk. In the α-tocopherol/β-carotene (ATBC) Lung Cancer Prevention Trial, participants were male smokers, age 50–69 years at entry. Participants had smoked an average of one pack of cigarettes per day for 35.9 years. Participants received α-tocopherol, β-carotene, and/or placebo in a randomized, two-by-two factorial design. After median follow-up of 6.1 years, lung cancer incidence and mortality were statistically significantly increased in those receiving β-carotene. α-Tocopherol had no effect on lung cancer mortality, and no evidence suggested interaction between the two drugs. Patients receiving α-tocopherol had a higher incidence of hemorrhagic stroke.
The β-Carotene and Retinol Efficacy Trial (CARET) involved 17,000 American smokers and workers with asbestos exposure. Entrants were randomly assigned to one of four arms and received β-carotene, retinol, and/or placebo in a two-by-two factorial design. This trial also demonstrated harm from β-carotene: a lung cancer rate of 5 per 1000 subjects per year for those taking placebo and of 6 per 1000 subjects per year for those taking β-carotene.
The ATBC and CARET results demonstrate the importance of testing chemoprevention hypotheses thoroughly before their widespread implementation because the results contradict a number of observational studies. The Physicians’ Health Trial showed no change in the risk of lung cancer for those taking β-carotene; however, fewer of its participants were smokers than those in the ATBC and CARET studies.
CHEMOPREVENTION OF COLON CANCER
Many colon cancer prevention trials are based on the premise that most colorectal cancers develop from adenomatous polyps. These trials use adenoma recurrence or disappearance as a surrogate endpoint (not yet validated) for colon cancer prevention. Early clinical trial results suggest that nonsteroidal anti-inflammatory drugs (NSAIDs), such as piroxicam, sulindac, and aspirin, may prevent adenoma formation or cause regression of adenomatous polyps. The mechanism of action of NSAIDs is unknown, but they are presumed to work through the cyclooxygenase pathway. Although two randomized controlled trials (the Physicians’ Health Study and the Women’s Health Study) did not show an effect of aspirin on colon cancer or adenoma incidence in persons with no previous history of colonic lesions after 10 years of therapy, these trials did show an approximately 18% relative risk reduction for colonic adenoma incidence in persons with a previous history of adenomas after 1 year. Pooled findings from observational cohort studies do demonstrate a 22% and 28% relative reduction in colorectal cancer and adenoma incidence, respectively, with regular aspirin use, and a well-conducted meta-analysis of four randomized controlled trials (albeit primarily designed to examine aspirin’s effects on cardiovascular events) found that aspirin at doses of at least 75 mg resulted in a 24% relative reduction in colorectal cancer incidence after 20 years, with no clear increase in efficacy at higher doses. Cyclooxygenase-2 (COX-2) inhibitors have also been considered for colorectal cancer and polyp prevention. Trials with COX-2 inhibitors were initiated, but an increased risk of cardiovascular events in those taking the COX-2 inhibitors was noted, suggesting that these agents are not suitable for chemoprevention in the general population.
Epidemiologic studies suggest that diets high in calcium lower colon cancer risk. Calcium binds bile and fatty acids, which cause proliferation of colonic epithelium. It is hypothesized that calcium reduces intraluminal exposure to these compounds. The randomized controlled Calcium Polyp Prevention Study found that calcium supplementation decreased the absolute risk of adenomatous polyp recurrence by 7% at 4 years; extended observational follow-up demonstrated a 12% absolute risk reduction 5 years after cessation of treatment. However, in the Women’s Health Initiative, combined use of calcium carbonate and vitamin D twice daily did not reduce the incidence of invasive colorectal cancer compared with placebo after 7 years.
The Women’s Health Initiative demonstrated that postmenopausal women taking estrogen plus progestin have a 44% lower relative risk of colorectal cancer compared to women taking placebo. Of >16,600 women randomized and followed for a median of 5.6 years, 43 invasive colorectal cancers occurred in the hormone group and 72 in the placebo group. The positive effect on colon cancer is mitigated by the modest increase in cardiovascular and breast cancer risks associated with combined estrogen plus progestin therapy.
A case-control study suggested that statins decrease the incidence of colorectal cancer; however, several subsequent case-control and cohort studies have not demonstrated an association between regular statin use and a reduced risk of colorectal cancer. No randomized controlled trials have addressed this hypothesis. A meta-analysis of statin use showed no protective effect of statins on overall cancer incidence or death.
CHEMOPREVENTION OF BREAST CANCER
Tamoxifen is an antiestrogen with partial estrogen agonistic activity in some tissues, such as endometrium and bone. One of its actions is to upregulate transforming growth factor β, which decreases breast cell proliferation. In randomized placebo-controlled trials to assess tamoxifen as adjuvant therapy for breast cancer, tamoxifen reduced the number of new breast cancers in the opposite breast by more than a third. In a randomized placebo-controlled prevention trial involving >13,000 pre- and postmenopausal women at high risk, tamoxifen decreased the risk of developing breast cancer by 49% (from 43.4 to 22 per 1000 women) after a median follow-up of nearly 6 years. Tamoxifen also reduced bone fractures; a small increase in risk of endometrial cancer, stroke, pulmonary emboli, and deep vein thrombosis was noted. The International Breast Cancer Intervention Study (IBIS-I) and the Italian Randomized Tamoxifen Prevention Trial also demonstrated a reduction in breast cancer incidence with tamoxifen use. A trial comparing tamoxifen with another selective estrogen receptor modulator, raloxifene, in postmenopausal women showed that raloxifene is comparable to tamoxifen in cancer prevention. This trial only included postmenopausal women. Raloxifene was associated with more invasive breast cancers and a trend toward more noninvasive breast cancers, but fewer thromboembolic events than tamoxifen; the drugs are similar in risks of other cancers, fractures, ischemic heart disease, and stroke. Both tamoxifen and raloxifene (the latter for postmenopausal women only) have been approved by the U.S. Food and Drug Administration (FDA) for reduction of breast cancer in women at high risk for the disease (1.66% risk at 5 years based on the Gail risk model: http://www.cancer.gov/bcrisktool/).
Because the aromatase inhibitors are even more effective than tamoxifen in adjuvant breast cancer therapy, it has been hypothesized that they would be more effective in breast cancer prevention. A randomized, placebo-controlled trial of exemestane reported a 65% relative reduction (from 5.5 to 1.9 per 1000 women) in the incidence of invasive breast cancer in women at elevated risk after a median follow-up of about 3 years. Common adverse effects included arthralgias, hot flashes, fatigue, and insomnia. No trial has directly compared aromatase inhibitors with selective estrogen receptor modulators for breast cancer chemoprevention.
CHEMOPREVENTION OF PROSTATE CANCER
Finasteride and dutasteride are 5-α-reductase inhibitors. They inhibit conversion of testosterone to dihydrotestosterone (DHT), a potent stimulator of prostate cell proliferation. The Prostate Cancer Prevention Trial (PCPT) randomly assigned men age 55 years or older at average risk of prostate cancer to finasteride or placebo. All men in the trial were being regularly screened with prostate-specific antigen (PSA) levels and digital rectal examination. After 7 years of therapy, the incidence of prostate cancer was 18.4% in the finasteride arm, compared with 24.4% in the placebo arm, a statistically significant difference. However, the finasteride group had more patients with tumors of Gleason score 7 and higher compared with the placebo arm (6.4 vs 5.1%). Reassuringly, long-term (10–15 years) follow-up did not reveal any statistically significant differences in overall mortality between all men in the finasteride and placebo arms or in men diagnosed with prostate cancer; differences in prostate cancer in favor of finasteride persisted.
Dutasteride has also been evaluated as a preventive agent for prostate cancer. The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial was a randomized double-blind trial in which approximately 8200 men with an elevated PSA (2.5–10 ng/mL for men age 50–60 years and 3–10 ng/mL for men age 60 years or older) and negative prostate biopsy on enrollment received daily 0.5 mg of dutasteride or placebo. The trial found a statistically significant 23% relative risk reduction in the incidence of biopsy-detected prostate cancer in the dutasteride arm at 4 years of treatment (659 cases vs 858 cases, respectively). Overall, across years 1 through 4, there was no difference between the arms in the number of tumors with a Gleason score of 7 to 10; however, during years 3 and 4, there was a statistically significant difference in tumors with Gleason score of 8 to 10 in the dutasteride arm (12 tumors vs 1 tumor, respectively).
The clinical importance of the apparent increased incidence of higher-grade tumors in the 5-α-reductase inhibitor arms of these trials is controversial. It may likely represent an increased sensitivity of PSA and digital rectal exam for high-grade tumors in men receiving these agents. The FDA has analyzed both trials, and it determined that the use of a 5-α-reductase inhibitor for prostate cancer chemoprevention would result in one additional high-grade (Gleason score 8 to 10) prostate cancer for every three to four lower-grade (Gleason score <6) tumors averted. Although it acknowledged that detection bias may have accounted for the finding, it stated that it could not conclusively dismiss a causative role for 5-α-reductase inhibitors. These agents are therefore not FDA-approved for prostate cancer prevention.
Because all men in both the PCPT and REDUCE trials were being screened and because screening approximately doubles the rate of prostate cancer, it is not known if finasteride or dutasteride decreases the risk of prostate cancer in men who are not being screened.
Several favorable laboratory and observational studies led to the formal evaluation of selenium and α-tocopherol (vitamin E) as potential prostate cancer preventives. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) assigned 35,533 men to receive 200 μg/d selenium, 400 IU/d α-tocopherol, selenium plus vitamin E, or placebo. After a median follow-up of 7 years, a trend toward an increased risk of developing prostate cancer was observed for those men taking vitamin E alone as compared to the placebo arm (hazard ratio 1.17; 95% confidence interval, 1.004–1.36).
VACCINES AND CANCER PREVENTION
A number of infectious agents cause cancer. Hepatitis B and C are linked to liver cancer; some HPV strains are linked to cervical, anal, and head and neck cancer; and Helicobacter pylori is associated with gastric adenocarcinoma and gastric lymphoma. Vaccines to protect against these agents may reduce the risk of their associated cancers.
The hepatitis B vaccine is effective in preventing hepatitis and hepatomas due to chronic hepatitis B infection.
A quadrivalent HPV vaccine (covering HPV strains 6, 11, 16, and 18) and a bivalent vaccine (covering HPV strains 16 and 18) are available for use in the United States. HPV types 16 and 18 cause cervical and anal cancer; reduction in these HPV types could prevent >70% of cervical cancers worldwide. HPV types 6 and 11 cause genital papillomas. For individuals not previously infected with these HPV strains, the vaccines demonstrate high efficacy in preventing persistent strain-specific HPV infections; however, the trials and substudies that evaluated the vaccines’ ability to prevent cervical and anal cancer relied on surrogate outcome measures (cervical or anal intraepithelial neoplasia [CIN/AIN] I, II, and III), and the degree of durability of the immune response beyond 5 years is not currently known. The vaccines do not appear to impact preexisting infections and the efficacy appears to be markedly lower for populations that had previously been exposed to vaccine-specific HPV strains. The vaccine is recommended in the United States for females and males age 9–26 years.
SURGICAL PREVENTION OF CANCER
Some organs in some individuals are at such high risk of developing cancer that surgical removal of the organ at risk may be considered. Women with severe cervical dysplasia are treated with laser or loop electrosurgical excision or conization and occasionally even hysterectomy. Colectomy is used to prevent colon cancer in patients with familial polyposis or ulcerative colitis.
Prophylactic bilateral mastectomy may be chosen for breast cancer prevention among women with genetic predisposition to breast cancer. In a prospective series of 139 women with BRCA1 and BRCA2 mutations, 76 chose to undergo prophylactic mastectomy and 63 chose close surveillance. At 3 years, no cases of breast cancer had been diagnosed in those opting for surgery, but eight patients in the surveillance group had developed breast cancer. A larger (n = 639) retrospective cohort study reported that three patients developed breast cancer after prophylactic mastectomy compared with an expected incidence of 30–53 cases: a 90–94% reduction in breast cancer risk. Postmastectomy breast cancer–related deaths were reduced by 81–94% for high-risk women compared with sister controls and by 100% for moderate-risk women when compared with expected rates.
Prophylactic oophorectomy may also be employed for the prevention of ovarian and breast cancers among high-risk women. A prospective cohort study evaluating the outcomes of BRCA mutation carriers demonstrated a statistically significant association between prophylactic oophorectomy and a reduced incidence of ovarian or primary peritoneal cancer (36% relative risk reduction, or a 4.5% absolute difference). Studies of prophylactic oophorectomy for prevention of breast cancer in women with genetic mutations have shown relative risk reductions of approximately 50%; the risk reduction may be greatest for women having the procedure at younger (i.e., <50 years) ages.
All of the evidence concerning the use of prophylactic mastectomy and oophorectomy for prevention of breast and ovarian cancer in high-risk women has been observational in nature; such studies are prone to a variety of biases, including case selection bias, family relationships between patients and controls, and inadequate information about hormone use. Thus, they may give an overestimate of the magnitude of benefit.
CANCER SCREENING
Screening is a means of detecting disease early in asymptomatic individuals, with the goal of decreasing morbidity and mortality. While screening can potentially reduce disease-specific deaths and has been shown to do so in cervical, colon, lung, and breast cancer, it is also subject to a number of biases that can suggest a benefit when actually there is none. Biases can even mask net harm. Early detection does not in itself confer benefit. Cause-specific mortality, rather than survival after diagnosis, is the preferred endpoint (see below).
Because screening is done on asymptomatic, healthy persons, it should offer substantial likelihood of benefit that outweighs harm. Screening tests and their appropriate use should be carefully evaluated before their use is widely encouraged in screening programs, as a matter of public policy.
A large and increasing number of genetic mutations and nucleotide polymorphisms have been associated with an increased risk of cancer. Testing for these genetic mutations could in theory define a high-risk population. However, most of the identified mutations have very low penetrance and individually provide minimal predictive accuracy. The ability to predict the development of a particular cancer may some day present therapeutic options as well as ethical dilemmas. It may eventually allow for early intervention to prevent a cancer or limit its severity. People at high risk may be ideal candidates for chemoprevention and screening; however, efficacy of these interventions in the high-risk population should be investigated. Currently, persons at high risk for a particular cancer can engage in intensive screening. While this course is clinically reasonable, it is not known if it reduces mortality in these populations.
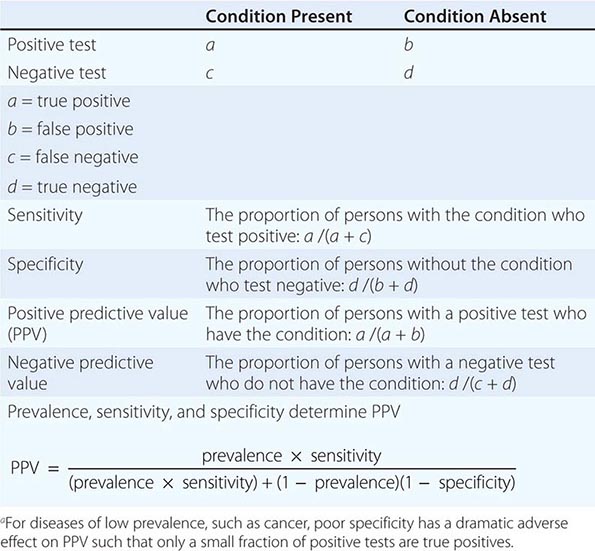
The Accuracy of Screening A screening test’s accuracy or ability to discriminate disease is described by four indices: sensitivity, specificity, positive predictive value, and negative predictive value (Table 100-2). Sensitivity, also called the true-positive rate, is the proportion of persons with the disease who test positive in the screen (i.e., the ability of the test to detect disease when it is present). Specificity, or 1 minus the false-positive rate, is the proportion of persons who do not have the disease that test negative in the screening test (i.e., the ability of a test to correctly identify that the disease is not present). The positive predictive value is the proportion of persons who test positive that actually have the disease. Similarly, negative predictive value is the proportion testing negative that do not have the disease. The sensitivity and specificity of a test are independent of the underlying prevalence (or risk) of the disease in the population screened, but the predictive values depend strongly on the prevalence of the disease.
ASSESSMENT OF THE VALUE OF A DIAGNOSTIC TESTa |
Screening is most beneficial, efficient, and economical when the target disease is common in the population being screened. Specificity is at least as important to the ultimate feasibility and success of a screening test as sensitivity.
Potential Biases of Screening Tests Common biases of screening are lead time, length-biased sampling, and selection. These biases can make a screening test seem beneficial when actually it is not (or even causes net harm). Whether beneficial or not, screening can create the false impression of an epidemic by increasing the number of cancers diagnosed. It can also produce a shift in the proportion of patients diagnosed at an early stage and inflate survival statistics without reducing mortality (i.e., the number of deaths from a given cancer relative to the number of those at risk for the cancer). In such a case, the apparent duration of survival (measured from date of diagnosis) increases without lives being saved or life expectancy changed.
Lead-time bias occurs whether or not a test influences the natural history of the disease; the patient is merely diagnosed at an earlier date. Survival appears increased even if life is not really prolonged. The screening test only prolongs the time the subject is aware of the disease and spends as a patient.
Length-biased sampling occurs because screening tests generally can more easily detect slow-growing, less aggressive cancers than fast-growing cancers. Cancers diagnosed due to the onset of symptoms between scheduled screenings are on average more aggressive, and treatment outcomes are not as favorable. An extreme form of length bias sampling is termed overdiagnosis, the detection of “pseudo disease.” The reservoir of some undetected slow-growing tumors is large. Many of these tumors fulfill the histologic criteria of cancer but will never become clinically significant or cause death. This problem is compounded by the fact that the most common cancers appear most frequently at ages when competing causes of death are more frequent.
Selection bias must be considered in assessing the results of any screening effort. The population most likely to seek screening may differ from the general population to which the screening test might be applied. In general, volunteers for studies are more health conscious and likely to have a better prognosis or lower mortality rate, irrespective of the screening result. This is termed the healthy volunteer effect.
Potential Drawbacks of Screening Risks associated with screening include harm caused by the screening intervention itself, harm due to the further investigation of persons with positive tests (both true and false positives), and harm from the treatment of persons with a true-positive result, whether or not life is extended by treatment (e.g., even if a screening test reduces relative cause-specific mortality by 20–30%, 70–80% of those diagnosed still go on to die of the target cancer). The diagnosis and treatment of cancers that would never have caused medical problems can lead to the harm of unnecessary treatment and give patients the anxiety of a cancer diagnosis. The psychosocial impact of cancer screening can also be substantial when applied to the entire population.
Assessment of Screening Tests Good clinical trial design can offset some biases of screening and demonstrate the relative risks and benefits of a screening test. A randomized controlled screening trial with cause-specific mortality as the endpoint provides the strongest support for a screening intervention. Overall mortality should also be reported to detect an adverse effect of screening and treatment on other disease outcomes (e.g., cardiovascular disease). In a randomized trial, two like populations are randomly established. One is given the usual standard of care (which may be no screening at all) and the other receives the screening intervention being assessed. The two populations are compared over time. Efficacy for the population studied is established when the group receiving the screening test has a better cause-specific mortality rate than the control group. Studies showing a reduction in the incidence of advanced-stage disease, improved survival, or a stage shift are weaker (and possibly misleading) evidence of benefit. These latter criteria are early indicators but not sufficient to establish the value of a screening test.
Although a randomized, controlled screening trial provides the strongest evidence to support a screening test, it is not perfect. Unless the trial is population-based, it does not remove the question of generalizability to the target population. Screening trials generally involve thousands of persons and last for years. Less definitive study designs are therefore often used to estimate the effectiveness of screening practices. However, every nonrandomized study design is subject to strong confounders. In descending order of strength, evidence may also be derived from the findings of internally controlled trials using intervention allocation methods other than randomization (e.g., allocation by birth date, date of clinic visit); the findings of analytic observational studies; or the results of multiple time series studies with or without the intervention.
Screening for Specific Cancers Screening for cervical, colon, and breast cancer is beneficial for certain age groups. Depending on age and smoking history, lung cancer screening can also be beneficial in specific settings. Special surveillance of those at high risk for a specific cancer because of a family history or a genetic risk factor may be prudent, but few studies have assessed the influence on mortality. A number of organizations have considered whether or not to endorse routine use of certain screening tests. Because these groups have not used the same criteria to judge whether a screening test should be endorsed, they have arrived at different recommendations. The American Cancer Society (ACS) and the U.S. Preventive Services Task Force (USPSTF) publish screening guidelines (Table 100-3); the American Academy of Family Practitioners (AAFP) generally follow/endorse the USPSTF recommendations; and the American College of Physicians (ACP) develops recommendations based on structured reviews of other organizations’ guidelines.
SCREENING RECOMMENDATIONS FOR ASYMPTOMATIC SUBJECTS NOT KNOWN TO BE AT INCREASED RISK FOR THE TARGET CONDITIONa |
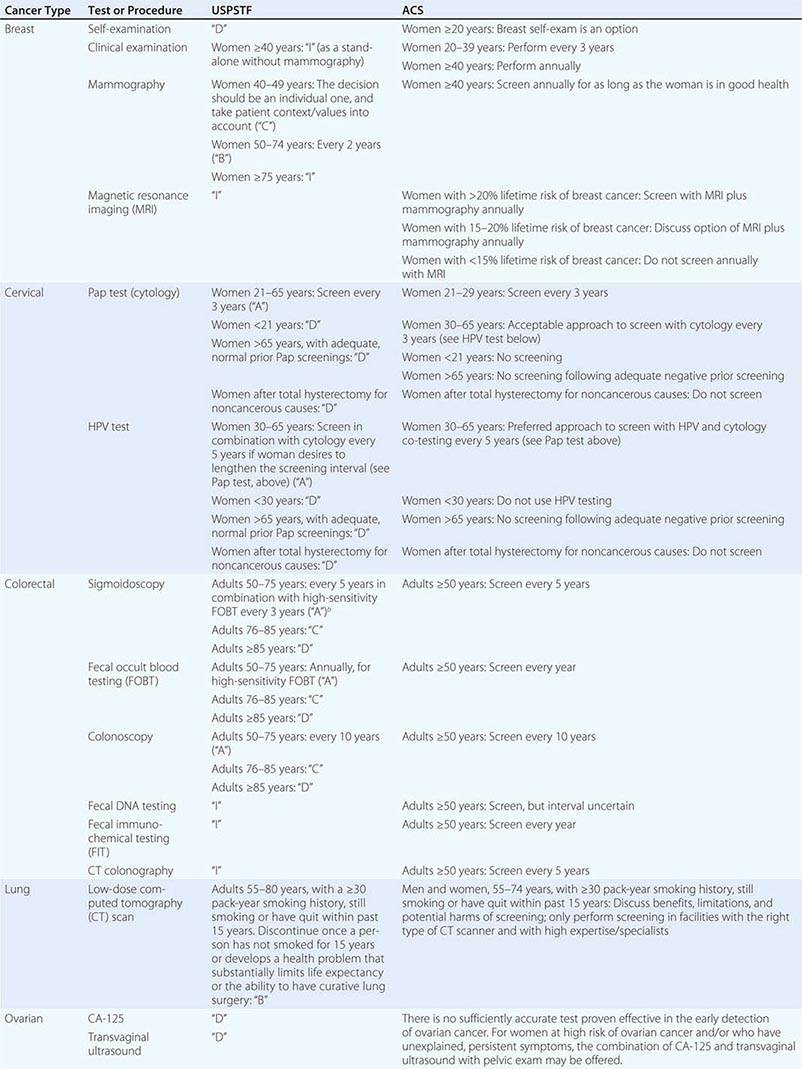

BREAST CANCER Breast self-examination, clinical breast examination by a caregiver, mammography, and magnetic resonance imaging (MRI) have all been variably advocated as useful screening tools.
A number of trials have suggested that annual or biennial screening with mammography or mammography plus clinical breast examination in normal-risk women older than age 50 years decreases breast cancer mortality. Each trial has been criticized for design flaws. In most trials, breast cancer mortality rate is decreased by 15–30%. Experts disagree on whether average-risk women age 40–49 years should receive regular screening (Table 100-3). The U.K. Age Trial, the only randomized trial of breast cancer screening to specifically evaluate the impact of mammography in women age 40–49 years, found no statistically significant difference in breast cancer mortality for screened women versus controls after about 11 years of follow-up (relative risk 0.83; 95% confidence interval 0.66–1.04); however, <70% of women received screening in the intervention arm, potentially diluting the observed effect. A meta-analysis of eight large randomized trials showed a 15% relative reduction in mortality (relative risk 0.85; 95% confidence interval 0.75–0.96) from mammography screening for women age 39–49 years after 11–20 years of follow-up. This is equivalent to a number needed to invite to screening of 1904 over 10 years to prevent one breast cancer death. At the same time, nearly half of women age 40–49 years screened annually will have false-positive mammograms necessitating further evaluation, often including biopsy. Estimates of overdiagnosis range from 10 to 40% of diagnosed invasive cancers. In the United States, widespread screening over the last several decades has not been accompanied by a reduction in incidence of metastatic breast cancer despite a large increase in early-stage disease, suggesting a substantial amount of overdiagnosis at the population level.
No study of breast self-examination has shown it to decrease mortality. A randomized controlled trial of approximately 266,000 women in China demonstrated no difference in breast cancer mortality between a group that received intensive breast self-exam instruction and reinforcement/reminders and controls at 10 years of follow-up. However, more benign breast lesions were discovered and more breast biopsies were performed in the self-examination arm.
Genetic screening for BRCA1 and BRCA2 mutations and other markers of breast cancer risk has identified a group of women at high risk for breast cancer. Unfortunately, when to begin and the optimal frequency of screening have not been defined. Mammography is less sensitive at detecting breast cancers in women carrying BRCA1 and BRCA2 mutations, possibly because such cancers occur in younger women, in whom mammography is known to be less sensitive. MRI screening may be more sensitive than mammography in women at high risk due to genetic predisposition or in women with very dense breast tissue, but specificity may be lower. An increase in overdiagnosis may accompany the higher sensitivity. The impact of MRI on breast cancer mortality with or without concomitant use of mammography has not been evaluated in a randomized controlled trial.
CERVICAL CANCER Screening with Papanicolaou (Pap) smears decreases cervical cancer mortality. The cervical cancer mortality rate has fallen substantially since the widespread use of the Pap smear. With the onset of sexual activity comes the risk of sexual transmission of HPV, the fundamental etiologic factor for cervical cancer. Screening guidelines recommend regular Pap testing for all women who have reached the age of 21 (prior to this age, even in individuals that have begun sexual activity, screening may cause more harm than benefit). The recommended interval for Pap screening is 3 years. Screening more frequently adds little benefit but leads to important harms, including unnecessary procedures and overtreatment of transient lesions. Beginning at age 30, guidelines also offer the alternative of combined Pap smear and HPV testing for women. The screening interval for women who test normal using this approach may be lengthened to 5 years.
An upper age limit at which screening ceases to be effective is not known, but women age 65 years with no abnormal results in the previous 10 years may choose to stop screening. Screening should be discontinued in women who have undergone a hysterectomy for noncancerous reasons.
Although the efficacy of the Pap smear in reducing cervical cancer mortality has never been directly confirmed in a randomized, controlled setting, a clustered randomized trial in India evaluated the impact of one-time cervical visual inspection and immediate colposcopy, biopsy, and/or cryotherapy (where indicated) versus counseling on cervical cancer deaths in women age 30–59 years. After 7 years of follow-up, the age-standardized rate of death due to cervical cancer was 39.6 per 100,000 person-years in the intervention group versus 56.7 per 100,000 person-years in controls.
COLORECTAL CANCER Fecal occult blood testing (FOBT), digital rectal examination (DRE), rigid and flexible sigmoidoscopy, colonoscopy, and computed tomography (CT) colonography have been considered for colorectal cancer screening. A meta-analysis of four randomized controlled trials demonstrated a 15% relative reduction in colorectal cancer mortality with FOBT. The sensitivity for FOBT is increased if specimens are rehydrated before testing, but at the cost of lower specificity. The false-positive rate for rehydrated FOBT is high; 1–5% of persons tested have a positive test. Only 2–10% of those with occult blood in the stool have cancer. The high false-positive rate of FOBT dramatically increases the number of colonoscopies performed.
Fecal immunochemical tests appear to have higher sensitivity for colorectal cancer than nonrehydrated FOBT tests. Fecal DNA testing is an emerging testing modality; it may have increased sensitivity and comparable specificity to FOBT and could potentially reduce harms associated with follow-up of false-positive tests. The body of evidence on the operating characteristics and effectiveness of fecal DNA tests in reducing colorectal cancer mortality is limited.
Two meta-analyses of five randomized controlled trials of sigmoidoscopy (i.e., the NORCCAP, SCORE, PLCO, Telemark, and U.K. trials) found an 18% relative reduction in colorectal cancer incidence and a 28% relative reduction in colorectal cancer mortality. Participant ages ranged from 50 to 74 years, with follow-up ranging from 6 to 13 years. Diagnosis of adenomatous polyps by sigmoidoscopy should lead to evaluation of the entire colon with colonoscopy. The most efficient interval for screening sigmoidoscopy is unknown, but an interval of 5 years is often recommended. Case-control studies suggest that intervals of up to 15 years may confer benefit; the U.K. trial demonstrated benefit with one-time screening.
One-time colonoscopy detects ∼25% more advanced lesions (polyps >10 mm, villous adenomas, adenomatous polyps with high-grade dysplasia, invasive cancer) than one-time FOBT with sigmoidoscopy; comparative programmatic performance of the two modalities over time is not known. Perforation rates are about 3/1000 for colonoscopy and 1/1000 for sigmoidoscopy. Debate continues on whether colonoscopy is too expensive and invasive and whether sufficient provider capacity exists to be recommended as the preferred screening tool in standard-risk populations. Some observational studies suggest that efficacy of colonoscopy to decrease colorectal cancer mortality is primarily limited to the left side of the colon.
CT colonography, if done at expert centers, appears to have a sensitivity for polyps ≥6 mm comparable to colonoscopy. However, the rate of extracolonic findings of abnormalities of uncertain significance that must nevertheless be worked up is high (∼15–30%); the long-term cumulative radiation risk of repeated colonography screenings is also a concern.
LUNG CANCER Chest x-ray and sputum cytology have been evaluated in several randomized lung cancer screening trials. The most recent and largest (n = 154,901) of these, one substudy of the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer screening trial, found that, compared with usual care, annual chest x-ray did not reduce the risk of dying from lung cancer (relative risk 0.99; 95% confidence interval 0.87–1.22) after 13 years. Low-dose CT has also been evaluated in several randomized trials. The largest and longest of these, the National Lung Screening Trial (NLST), was a randomized controlled trial of screening for lung cancer in approximately 53,000 persons age 55–74 years with a 30+ pack-year smoking history. It demonstrated a statistically significant relative reduction of about 15–20% in lung cancer mortality in the CT arm compared to the chest x-ray arm (or about 3 fewer deaths per 1000 people screened with CT). However, the harms include the potential radiation risks associated with multiple scans, the discovery of incidental findings of unclear significance, and a high rate of false-positive test results. Both incidental findings and false-positive tests can lead to invasive diagnostic procedures associated with anxiety, expense, and complications (e.g., pneumo- or hemothorax after lung biopsy). The NLST was performed at experienced screening centers, and the balance of benefits and harms may differ in the community setting at less experienced centers.
OVARIAN CANCER Adnexal palpation, transvaginal ultrasound (TVUS), and serum CA-125 assay have been considered for ovarian cancer screening. A large randomized controlled trial has shown that an annual screening program of TVUS and CA-125 in average-risk women does not reduce deaths from ovarian cancer (relative risk 1.21; 95% confidence interval 0.99–1.48). Adnexal palpation was dropped early in the study because it did not detect any ovarian cancers that were not detected by either TVUS or CA-125. The risks and costs associated with the high number of false-positive results are impediments to routine use of these modalities for screening. In the PLCO trial, 10% of participants had a false-positive result from TVUS or CA-125, and one-third of these women underwent a major surgical procedure; the ratio of surgeries to screen-detected ovarian cancer was approximately 20:1.
PROSTATE CANCER The most common prostate cancer screening modalities are DRE and serum PSA assay. An emphasis on PSA screening has caused prostate cancer to become the most common nonskin cancer diagnosed in American males. This disease is prone to lead-time bias, length bias, and overdiagnosis, and substantial debate continues among experts as to whether screening should be offered unless the patient specifically asks to be screened. Virtually all organizations stress the importance of informing men about the uncertainty regarding screening efficacy and the harms associated with screening. Prostate cancer screening clearly detects many asymptomatic cancers, but the ability to distinguish tumors that are lethal but still curable from those that pose little or no threat to health is limited, and randomized trials indicate that the effect of PSA screening on prostate cancer mortality across a population is, at best, small. Men older than age 50 years have a high prevalence of indolent, clinically insignificant prostate cancers (about 30–50% of men, increasing further as men age).
Two major randomized controlled trials of the impact of PSA screening on prostate cancer mortality have been published. The PLCO Cancer Screening Trial was a multicenter U.S. trial that randomized almost 77,000 men age 55–74 years to receive either annual PSA testing for 6 years or usual care. At 13 years of follow-up, no statistically significant difference in the number of prostate cancer deaths were noted between the arms (rate ratio 1.09; 95% confidence interval 0.87–1.36). Approximately 50% of men in the control arm received at least one PSA test during the trial, which may have potentially diluted a small effect.
The European Randomized Study of Screening for Prostate Cancer (ERSPC) was a multinational study that randomized approximately 182,000 men between age 50 and 74 years (with a predefined “core” screening group of men age 55–69 years) to receive PSA testing or no screening. Recruitment and randomization procedures, as well as actual frequency of PSA testing, varied by country. After a median follow-up of 11 years, a 20% relative reduction in the risk of prostate cancer death in the screened arm was noted in the “core” screening group. The trial found that 1055 men would need to be invited to screening, and 37 cases of prostate cancer detected, to avert 1 death from prostate cancer. Of the seven countries included in the mortality analysis, two demonstrated statistically significant reductions in prostate cancer deaths, whereas five did not. There was also an imbalance in treatment between the two study arms, with a higher proportion of men with clinically localized cancer receiving radical prostatectomy in the screening arm and receiving it at experienced referral centers.
Treatments for low-stage prostate cancer, such as surgery and radiation therapy, can cause significant morbidity, including impotence and urinary incontinence. In a trial conducted in the United States after the initiation of widespread PSA testing, random assignment to radical prostatectomy compared with “watchful waiting” did not result in a statistically significant decrease in prostate cancer deaths (absolute risk reduction 2.7%; 95% confidence interval–1.3 to 6.2%).
SKIN CANCER Visual examination of all skin surfaces by the patient or by a health care provider is used in screening for basal and squamous cell cancers and melanoma. No prospective randomized study has been performed to look for a mortality decrease. Unfortunately, screening is associated with a substantial rate of overdiagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree