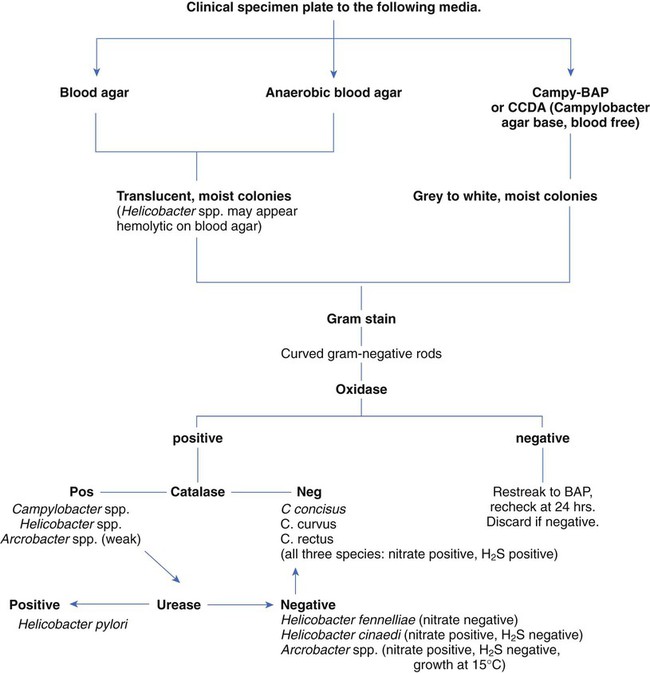
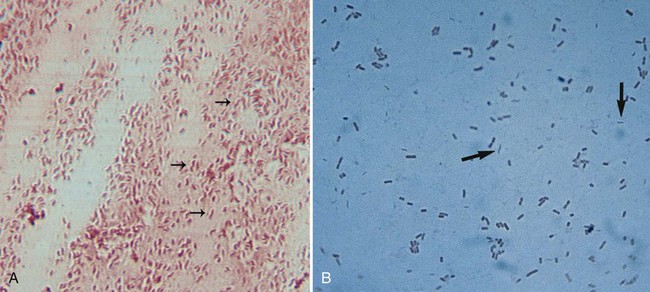
1. List the Campylobacter species most often associated with infections in humans, and explain how they are transmitted. 2. Identify the culture methods for optimum recovery of Campylobacter jejuni and Campylobacter coli, including agar, temperatures, oxygenation, and length of incubation. 3. Describe how to isolate Campylobacter from blood, including special stains, atmospheric conditions, and length of incubation. 4. List the colonial morphology, microscopic, and biochemical reactions of Campylobacter and Helicobacter. 5. List the key biochemical test to identify Helicobacter pylori in specimens. 6. Describe how H. pylori colonize in the stomach and how motility plays an important role in the pathogenesis of the organism. Because of morphologic similarities and an inability to recover these organisms using routine laboratory media for primary isolation, the genera Campylobacter, Arcobacter, and Helicobacter are considered in this chapter (Figure 34-1). All organisms belonging to these genera are small, curved, motile, gram-negative bacilli. With few exceptions, most of these bacteria also have a requirement for a microaerobic (5% to 10% O2) atmosphere. Campylobacter and Arcobacter spp. are relatively slow growing, fastidious, and, in general, asaccharolytic; organisms known to cause disease in humans are listed in Table 34-1. TABLE 34-1 Campylobacter and Arcobacter spp., Their Source, and Spectrum of Disease in Humans As previously mentioned, Campylobacter species are the causative agent of gastrointestinal or extraintestinal infections. An increase in extraintestinal disease, including meningitis, endocarditis, and septic arthritis has been reported in patients with acquired immunodeficiency syndrome (AIDS) and other immunocompromised individuals. The different campylobacters and the associated diseases are summarized in Table 34-1. Gastroenteritis associated with Campylobacter spp. is usually a self-limiting illness and does not require antibiotic therapy. Most recently, postinfectious complications with C. jejuni have been recognized and include reactive arthritis and Guillain-Barré syndrome, an acute demyelination (removal of the myelin sheath from a nerve) of the peripheral nerves. Studies indicate that 20% to 40% of patients with this syndrome are infected with C. jejuni 1 to 3 weeks prior to the onset of neurologic symptoms. Upon gram staining, Campylobacter spp. display a characteristic microscopic morphology as small, curved or seagull-winged, faintly staining, gram-negative rods (Figure 34-2). Polymerase chain reaction (PCR) amplification may provide an alternative to culture methods for the detection of Campylobacter spp. from clinical specimens. The detection of Campylobacter DNA in stools from a large number of patients with diarrhea suggests that Campylobacter spp. other than C. jejuni and C. coli may account for a proportion of cases of acute gastroenteritis in which no etiologic agent is identified. Successful isolation of Campylobacter spp. from stool requires selective media and optimum incubation conditions. Recommended inoculation of two selective agars is associated with increased recovery of the organisms. Because Campylobacter and Arcobacter spp. have different optimum temperatures, two sets of selective plates should be incubated, one at 42° C and one at 37° C. Extended incubation may be required, 48 to 72 hours, before there is evidence of visible growth. Table 34-2 describes the selective plating media and incubation conditions required for the recovery of Campylobacter spp. from stool specimens. TABLE 34-2 Modified Skirrow’s medium: Columbia blood agar base, 7% horse-lysed blood, and antibiotics (vancomycin, trimethoprim, and polymyxin B) Campy-BAP: Brucella agar base with antibiotics (trimethoprim, polymyxin B, cephalothin, vancomycin, and amphotericin B) and 10% sheep blood Blood-free, charcoal-based selective medium: Columbia base with charcoal, hemin, sodium pyruvate, and antibiotics (vancomycin, cefoperazone, and cycloheximide) Modified charcoal cefoperazone deoxycholate agar (CCDA) Semisolid motility agar: Mueller-Hinton broth II, agar, cefoperazone, and trimethoprim lactate Campy-CVA: Brucella agar base with antibiotics (cefoperazone, vancomycin, and amphotericin B) and 5% sheep blood *Atmosphere can be generated in several ways, including commercially produced, gas-generating envelopes to be used with plastic bags or jars. Evacuation and replacement in plastic bags or anaerobic jars with an atmosphere of 10% CO2, 5% O2, and the balance of nitrogen (N2) is the most cost-effective method, although it is labor intensive. †All these organisms are susceptible to cephalothin. ‡C. upsaliensis will grow at 42° C but not on cephalothin-containing selective agar.
Campylobacter, Arcobacter, and Helicobacter
Campylobacter and Arcobacter
General Characteristics
Organism
Source
Spectrum of Disease in Humans
C. concisus, C. curvus, C. rectus, C. showae
Humans
Periodontal disease; gastroenteritis (?)
C. gracilis
Humans
Deep-tissue infections: head, neck, and viscera; gingival crevices
C. coli
Pigs, poultry, sheep, bulls, birds
C. jejuni subsp. jejuni
Poultry, pigs, bulls, dogs, cats, birds, and other animals
C. jejuni subsp. doylei
Humans
C. lari
Birds, poultry, other animals; river and seawater
C. hyointestinalis subsp. hyointestinalis
Pigs, cattle, hamsters, deer
Gastroenteritis
C. upsaliensis
Dogs, cats
C. fetus subsp. fetus
Cattle, sheep
C. fetus subsp. venerealis
Cattle
Septicemia
C. sputorum biovar sputorum
Humans, cattle, pigs
Arcobacter cryaerophilus
Pigs, bulls, and other animals
A. butzleri
Pigs, bulls, humans, other animals; water
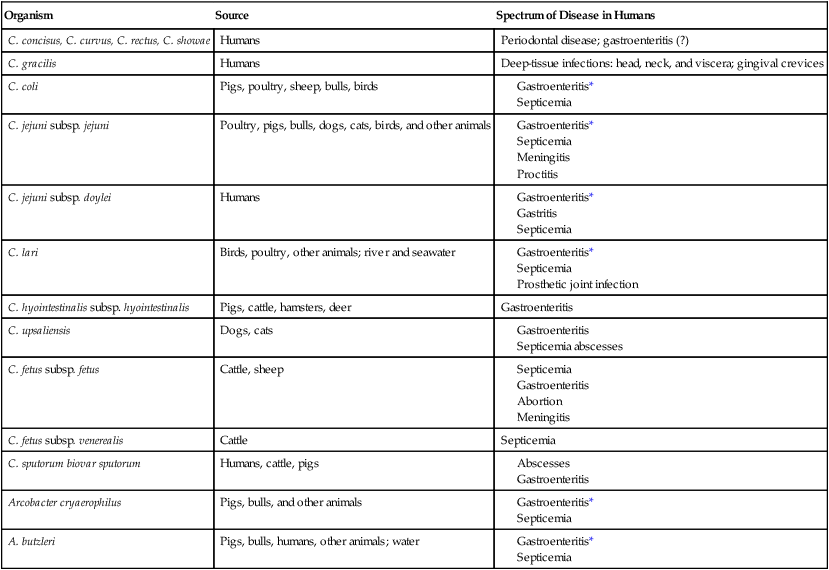
Spectrum of Disease
Laboratory Diagnosis
Specimen Collection, Transport, and Processing
Direct Detection
Cultivation
Stool.
Organism
Primary Plating Media
Incubation Conditions
42° C microaerobic conditions* for 72 hours
37° C under microaerobic conditions for at least 72 hours up to 7 days‡
A. cryaerophilus, A. butzleri
Campy-CVA
37° C under microaerobic conditions§ for 72 hours

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree