, Arthur H. Cohen2, Robert B. Colvin3, J. Charles Jennette4 and Charles E. Alpers5
(1)
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, Tennessee, USA
(2)
Department of Pathology and Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, California, USA
(3)
Department of Pathology Harvard Medical School, Massachusetts General Hospital, Boston, Massachusetts, USA
(4)
Department of Pathology and Laboratory Medicine, University of North Carolina, Chapel Hill, North Carolina, USA
(5)
Department of Pathology, University of Washington, Seattle, Washington, USA
Abstract
Cyclosporin A (CsA) and tacrolimus have greatly improved graft survival since their introduction in the 1980 and 1990s, respectively. While the drugs are structurally unrelated, their mechanism of immunosuppression is remarkably similar. The dramatic immunosuppressive and nephrotoxic effects of CsA and tacrolimus are largely explained by their calcineurin inhibition. The pathology of CsA and tacrolimus toxicity is pathologically indistinguishable.
Calcineurin Inhibitor Toxicity (CIT)
Introduction/Clinical Setting
Cyclosporin A (CsA) and tacrolimus have greatly improved graft survival since their introduction in the 1980 and 1990s, respectively. While the drugs are structurally unrelated, their mechanism of immunosuppression is remarkably similar. The dramatic immunosuppressive and nephrotoxic effects of CsA and tacrolimus are largely explained by their calcineurin inhibition. The pathology of CsA and tacrolimus toxicity is pathologically indistinguishable.
Pathologic Findings
There are three pathologic forms of toxicity: acute nephrotoxicity, chronic nephrotoxicity, and thrombotic microangiopathy (hemolytic-uremic syndrome). Each can also arise in native kidneys in patients on CsA or tacrolimus for other reasons.
Acute Tubulopathy
The biopsy features of acute toxicity range from no morphologic abnormality to acute tubular injury or marked tubular vacuolization and vascular smooth muscle apoptosis (Fig. 21.1). The proximal tubules show loss of brush borders and isometric clear vacuolization (defined as cells filled with uniformly sized vacuoles). The vacuoles, much smaller than the nucleus, contain clear aqueous fluid rather than lipid and are indistinguishable from those caused by osmotic diuretics. Immunofluorescence microscopy is negative. By electron microscopy, the vacuoles are dilated endoplasmic reticulum and appear empty [1]. These lesions are reversible with decreased dosage.
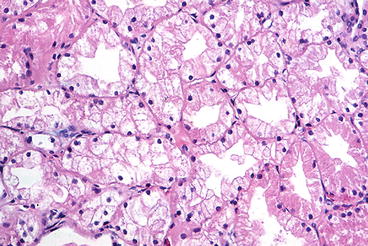

Fig. 21.1
Acute calcineurin inhibitor toxicity, showing isometric vacuolization of proximal tubules (H&E stain)
Arteriolopathy
A spectrum of acute and chronic arteriolopathy has been described by Mihatsch, ranging from acute, focal myocyte necrosis and mucoid intimal thickening to indolent nodular hyaline deposits (Fig. 21.2) [2]. The characteristic features are individual smooth muscle cell degeneration, vacuolization, necrosis, and loss. The myocytes are replaced by hyaline deposits, which are classically in a beaded pattern in the media.
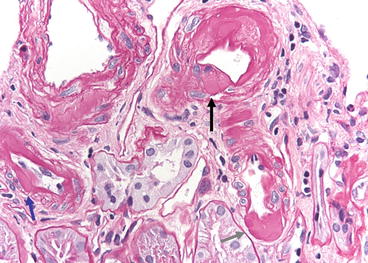

Fig. 21.2
Chronic calcineurin inhibitor toxicity. Peripheral nodules of hyaline are in an arteriole (black arrow). Conventional subendothelial hyaline is present (blue arrow), as well as advanced transmural hyaline deposits (gray arrow). PAS stain
The usual hyaline deposits in hypertension or diabetes are subendothelial or transmural. The endothelial or smooth muscle cells may be vacuolated. Later biopsies show progressive scarring of arterioles, intimal fibrosis, and segmental glomerular obsolescence. Immunofluorescence of early lesions may show that the vessels have deposits of immunoglobulin M (IgM), C3, and fibrin. Electron microscopy shows apoptosis or necrosis of smooth muscle cells and replacement with hyaline material. Focal myocyte necrosis in the media of small arteries, in the absence of intimal changes, is regarded as a reliable indicator of CsA toxicity [3]. However, while nodular peripheral hyaline deposits are more prevalent in biopsies from patients on CNI, they also occur with some frequency in patients who have never received these drugs [4].
Thrombotic Microangiopathy
Although more prevalent with higher doses of CsA in the 1980s, thrombotic microangiopathy (Fig. 21.3) still occurs under current regimens, even with careful attention to blood CsA levels. By 1994, the prevalence of CsA-associated thrombotic microangiopathy had decreased to 0.9 %, accounting for 26 % of the cases of thrombotic microangiopathy after renal transplantation (acute rejection, probably humoral, accounted for 53 % and recurrent thrombotic microangiopathy 16 %) [5]. Patients typically present with acute renal failure, thrombocytopenia, microangiopathic hemolytic anemia, elevated lactic dehydrogenase, and hyperbilirubinemia. Despite these characteristic features, the clinical syndrome is not often recognized before biopsy. Those without systemic signs (thrombocytopenia, hemolysis) do considerably better [6].
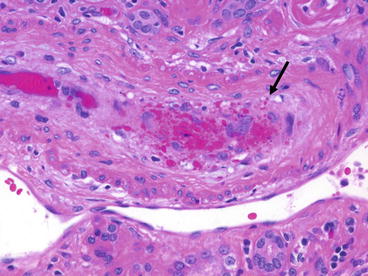

Fig. 21.3
Acute calcineurin inhibitor toxicity due to tacrolimus. Marked mucoid intimal thickening is present with a sparse mononuclear infiltrate and numerous trapped red cell fragments (arrow)
The pathologic changes are the same as in thrombotic microangiopathy from other causes, although in the allograft the differential diagnosis with endarteritis can be challenging. Loose intimal thickening and trapped red cells are useful discriminators.
Differential Diagnosis
The criteria for the morphologic distinction of calcineurin inhibitor toxicity (CIT) and rejection have received much attention. Interstitial infiltrates are minimal in autologous kidneys with nephrotoxicity, but are common in early allografts, and have no differential value unless minimal. Patients with rejection typically have a diffuse, interstitial mononuclear cell infiltrate, whereas patients with CIT and those with stable function have only focal mononuclear cell infiltrates [7]. Endarteritis is found rarely, if ever, in CIT (0–1 %) and is the most discriminating feature between acute rejection and CIT. Only the finding of endarteritis allowed the identification of rejection with any certainty [8]. This agrees with my experience and that of others [3]. Endothelial and medial smooth muscle cell vacuolization has been noted in CIT, best appreciated by electron microscopy. The frequency of vacuolization probably does not distinguish CIT from stable grafts [7].
Polyomavirus
Introduction/Clinical Setting
The BK polyomavirus was originally isolated from B. K., a Sudanese patient who had distal donor ureteral stenosis months after a living related transplant [9]. BK virus is related to JC virus (which also inhabits the human urinary tract) and to simian kidney virus SV-40. These viruses are members of the papovavirus group, which includes the papillomaviruses. The BK virus commonly infects urothelium but rarely causes morbidity in immunocompetent individuals. However, in renal transplant recipients three lesions have been attributed to BK virus: hemorrhagic cystitis, ureteral stenosis, and acute interstitial nephritis [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


