Breast-Conserving Surgery
J. Michael Dixon
The aim of local treatment of breast cancer is to achieve long-term local disease control with the minimum of local morbidity. The majority of women who present to breast clinics with symptoms or who are diagnosed as having breast cancer through screening programs have small breast cancers that are suitable for breast-conserving surgery. The major advantages of breast-conserving treatment (BCT) are that it produces
an acceptable cosmetic appearance in the majority of women
lower levels of psychological morbidity with less anxiety, depression, improved body image, sexuality, and self-esteem than mastectomy
equivalence in terms of disease outcome for BCT and mastectomy, as shown by two systematic reviews
One of these reviews analyzed data from six randomized controlled trials comparing BCT with mastectomy. A meta-analysis of data from five of these six trials, which included 3,006 women, found no significant difference in the risk of death at 10 years (odds ratio, 0.91, 95% confidence interval (CI), 0.78 to 1.05). The sixth randomized trial used different protocols. In the second systematic review, nine randomized control trials involving 4,981 women randomized to mastectomy or BCT were included in the analysis. A meta-analysis of these nine trials found no significant difference in the risk of death over 10 years (relative risk reduction [RRR] for breast-conserving surgery when compared with mastectomy was 0.02, 95% CI -0.05 to +0.09). There was also no difference in the rates of local recurrence in the six randomized control trials involving 3,107 women for whom data were available (RRR mastectomy vs. BCT, 0.04, 95% CI -0.04 to +0.12). The Milan group (Veronesi et al.) published their 20-year follow-up results and, although they showed a significant difference in local recurrence within the ipsilateral breast of 2.3% in the mastectomy group and 8.8% in the BCT group, the disease-specific survival was similar between the two groups, with rates of death from breast cancer being 24.3% in the mastectomy group and 26.1% in the BCT group.
Originally it was thought that local therapy had little influence on overall survival, but it is becoming clear that local therapy is responsible, at least in part, for some patients developing metastatic disease. It is important, therefore, to ensure that only appropriate patients are selected for BCT. For those patients who have this treatment, the aim is to minimize local recurrence and at the same time achieve a good cosmetic outcome.
Table 1 Indications and Contraindications for Breast-Conserving Surgery | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Selection of Patients for Breast Conservation
Clinically, solitary cancers measuring 4 cm or less, without signs of involvement of skin or chest wall, can usually be managed by BCT (Table 1). Many units consider tumors measuring 3 cm or less clinically as the
maximum size of a tumor for a patient to be able to undergo BCT. Increasing tumor size, however, does not mean increasing local recurrence rates, and this approach is therefore illogical. Clinical tumor size overestimates actual tumor size. There is a much better correlation between pathologic tumor size and the size measured on imaging, with ultrasound and magnetic resonance imaging assessment being more accurate than mammographic measurements. It is the balance between tumor size as assessed by imaging and breast volume that determines whether a patient is suitable for BCT. Patients with tumors measuring clinically larger than 4 cm can be treated by BCT if the patient has large breasts. Conversely, in a patient with small breasts, excision of even a 1-cm tumor may produce an unacceptable cosmetic result. Options for patients with tumors considered too large relative to the size of the breast for BCT include neoadjuvant systemic therapy to shrink the tumor or an oncoplastic procedure, involving either transfer of tissue into the breast or surgery to reduce the size of the opposite breast to obtain symmetry.
maximum size of a tumor for a patient to be able to undergo BCT. Increasing tumor size, however, does not mean increasing local recurrence rates, and this approach is therefore illogical. Clinical tumor size overestimates actual tumor size. There is a much better correlation between pathologic tumor size and the size measured on imaging, with ultrasound and magnetic resonance imaging assessment being more accurate than mammographic measurements. It is the balance between tumor size as assessed by imaging and breast volume that determines whether a patient is suitable for BCT. Patients with tumors measuring clinically larger than 4 cm can be treated by BCT if the patient has large breasts. Conversely, in a patient with small breasts, excision of even a 1-cm tumor may produce an unacceptable cosmetic result. Options for patients with tumors considered too large relative to the size of the breast for BCT include neoadjuvant systemic therapy to shrink the tumor or an oncoplastic procedure, involving either transfer of tissue into the breast or surgery to reduce the size of the opposite breast to obtain symmetry.
Because of an initially high reported incidence of in breast recurrence, patients with multiple tumors in the same breast had not been previously considered good candidates for BCT. This view has changed and provided that the cancers can be excised to clear margins even if they are far apart and a satisfactory cosmetic outcome produced, BCT is feasible. In some patients who have large areas of the breast involved, BCT may be possible if both breasts are made smaller by a bilateral mammoplasty procedure. If there is widespread ductal carcinoma in situ (DCIS) alone or associated with an invasive cancer then most of these women are best treated by mastectomy, combined with immediate reconstruction in appropriate patients. Patients with two tumors close to each other visible on mammograms, or who have multifocal disease identified only by the pathologist, are very good candidates for BCT provided that all disease can be excised and the cosmetic outcome is not compromised. Patients with bilateral small cancers can also be treated by bilateral breast conservation.
Clinical and pathologic factors have previously influenced selection of patients for BCT because of their perceived impact on local recurrence. They are no longer considered as contraindications for BCT. These include young age (less than 35 to 39 years), the presence of an extensive in situ component associated with an invasive tumor, grade 3 histology, and widespread lymphatic/vascular invasion. One relative contraindication to BCT is the presence of a collagen vascular disease, as radiotherapy may create problems in such women. Central tumors were also initially a relative contraindication to BCT as the cosmetic result can be suboptimal. Most patients with small central tumors, however, are candidates for wide local excision with or without nipple/areolar excision and/or rotational flap advancement as described later in this chapter. Young women with a strong family history of breast cancer or known carriers of BRCA1 or BRCA2 mutations may be better served by bilateral mastectomy instead of BCT as they are at higher risk of developing either a recurrent cancer or a new cancer in the treated or opposite breast.
Patients are staged clinically according to the International Union against Cancer TNM (Tumor, Nodes, Metastasis) classification although none of the staging methods are of particular value in breast cancer. Clinical assessment of tumor size is not accurate and usually overestimates tumor size, although there is a correlation between clinical tumor size and final pathologic measurement. Clinical examination is unreliable in determining whether the axillary nodes are involved with metastatic breast cancer.
In early operable breast cancer (T l to 2, N0 to 1), there is no current evidence to support routine screening for metastatic disease in asymptomatic women. Patients with symptoms suggestive of metastases at a particular site require appropriate investigation. The incidence of asymptomatic metastases increases as the T and N stage increases. If it will affect treatment, patients with more advanced but operable disease (T3 and extensive N1 disease clinically or on imaging) should be considered for investigations to exclude distant metastases.
All patients with a breast mass should have a careful clinical examination and the mass should be measured with calipers. The presence or absence of any signs of local advancement such as inflammation, peau d’orange, ulceration, satellite nodules, direct chest wall involvement, and fixed axillary nodes should be noted and recorded. All patients should have good-quality bilateral mammograms with magnification views of any visible indeterminate calcification. Ultrasound of the whole breast is recommended to assess size of the tumor and ultrasound of the axilla should be performed to assess presence of metastatic lymph nodes and allow ultrasound-guided fine-needle aspiration biopsy or core biopsy of any lymph nodes with features suggestive of metastatic spread. Routine use of breast MRI is unnecessary but in certain patients it can provide information on disease extent.
The patient’s mammogram should be inspected before operation and excision planned to include the whole of the abnormal area. Some invasive carcinomas have surrounding noninvasive disease that is visible as microcalcification on mammograms. These microcalcifications should be excised and may require preoperative localization with a hook wire.
Informed consent should be obtained from the patient. As part of this procedure, the patient should be given information leaflets that describe the operation and outline common recognized potential complications.
Technique of Wide Local Excision
Two breast-conservation surgical procedures have been studied extensively and described: quadrantectomy and wide local excision. Quadrantectomy is based on the belief that the breast is organized into segments, with each segment draining into its own major duct, and that invasive cancer spreads down the duct system toward the nipple. The evidence is that both of these premises are incorrect. Studies have also shown that both invasive and noninvasive disease is no more likely to extend toward the nipple than in any other direction. The effectiveness of quadrantectomy relates to the large amount of tissue excised around the tumor rather than to the removal of a cancer and its draining duct. One of the early studies of BCT (Veronesi et al.) randomized patients to lumpectomy or quadrantectomy. A significantly greater number of patients who had lumpectomy had incomplete local excisions. Not surprisingly, therefore, local recurrence was greater after lumpectomy than after the more extensive quadrantectomy, although survival was no different. Other nonrandomized studies have shown similar rates of local recurrence following both quadrantectomy and wide local excision, provided margins of excision are clear. Quadrantectomy is no longer advocated because it removes substantially more breast tissue and so produces a significantly poorer cosmetic outcome than wide local excision. The consensus view is that the majority of patients having BCT can be adequately treated by wide local excision and do not require the more extensive excision of a quadrantectomy.
The majority of wide excisions of palpable and impalpable cancers are performed under general anesthesia, although it is possible to perform wide excision under local anesthesia
if the patient is unfit to have a general anesthetic or has a strong preference for local anesthesia. A meta-analysis of studies of prophylactic antibiotics in breast surgery has shown a small but significant reduction in postoperative infections if intravenous antibiotics are administered during induction of general anesthesia, so many surgeons give a dose of intravenous antibiotics to cover the operative period. Our practice is to use 1.2 g of co-amoxiclav or a cephalosporin. The lack of a clear advantage for antibiotics in randomized studies means that not all surgeons give them.
if the patient is unfit to have a general anesthetic or has a strong preference for local anesthesia. A meta-analysis of studies of prophylactic antibiotics in breast surgery has shown a small but significant reduction in postoperative infections if intravenous antibiotics are administered during induction of general anesthesia, so many surgeons give a dose of intravenous antibiotics to cover the operative period. Our practice is to use 1.2 g of co-amoxiclav or a cephalosporin. The lack of a clear advantage for antibiotics in randomized studies means that not all surgeons give them.
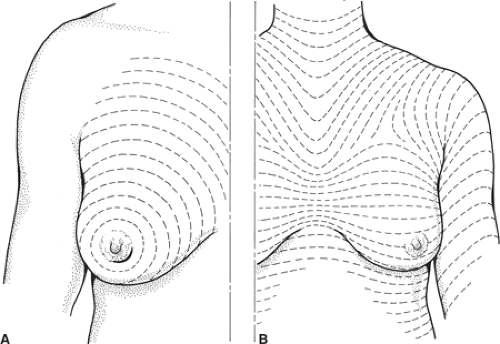 Fig. 1. The direction of Langer’s lines (A) and the lines of maximum resting skin tension in the breast (the so-called dynamic lines of Kraissl) (B). |
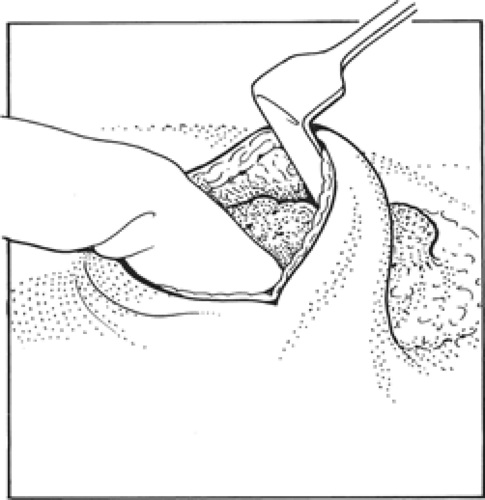 Fig. 2. Finger of nondominant hand is placed over palpable cancer and breast tissue is divided beyond the fingertips. |
Incisions
The aim of wide local excision is to remove all invasive and any ductal carcinoma in situ with a 1-cm macroscopic margin of normal surrounding breast tissue. It is important to place the incision in a position that will obtain the optimal cosmetic result. Langer described the predominant orientation of collagen fibers in the skin and around the breast, and these lines are essentially circular (Fig. 1A). Kraissl later demonstrated that lines of maximum resting skin tension run in a more transverse orientation across the breast (Fig. 1B). In general, scars that are parallel both to the lines of maximum resting skin tension and to the orientation of collagen fibers produce the best cosmetic incisions with least hypertrophy and keloid formation. Kraissl’s lines are more important and transverse incisions within the skin creases produce the best cosmetic results. Conversely, incisions that are at right angles to both the orientation of collagen fibers and the lines of maximum resting skin tension, such as radial incisions in the upper outer quadrant, produce the worst cosmetic results. Incisions to excise a cancer used to be placed directly over the lesion but increasingly remote incisions, including submammary, circumareolar, and axillary incisions, are being used as they produce superior cosmetic outcomes. Excising skin directly overlying a cancer is only necessary if the carcinoma is very superficial and/or the skin is tethered. It should not be performed routinely.
The cosmetic result after BCT is influenced by the amount of skin excised, with poor results being obtained in those patients who have most skin removed. Limiting the length of the incision is also important as longer incisions produce significantly poorer cosmetic outcomes.
After making the skin incision, the skin and subcutaneous fat are dissected off the breast tissue. This can be facilitated in remote incisions by hydrodissection injecting a 1 in 400,000 adrenaline solution in saline into the plane between the breast and subcutaneous fat. When elevating skin, it is important not to disrupt the subcutaneous fat as thin skin flaps give a poor cosmetic result. The skin flaps should be elevated 1 to 2 cm beyond the edge of the cancer. The fingers of the nondominant hand are then placed over the palpable cancer and the breast tissue divided beyond the fingertips (Fig. 2); the line of incision should be 1 cm beyond the limit of the palpable mass. Having divided breast tissue beyond the edge of the cancer, the deep aspect of the tumor can be palpated and breast tissue under the cancer divided. It is usually but not always necessary to remove full thickness of breast tissue. To ensure that there is an adequate margin deep to the cancer, for the majority of patients dissection through the breast tissue is continued down to the pectoral fascia and the breast tissue containing the cancer is lifted off the pectoral fascia. It is not necessary to excise pectoral fascia unless it is tethered to the tumor. If a carcinoma is infiltrating one of the chest wall muscles, then a portion of the affected muscle should be removed beneath the tumor in order to excise tissue beyond the limits of the cancer. For very superficial cancers in parts of the breast with significant depth, it is possible to clear the deep margins without removing the full thickness of the breast.
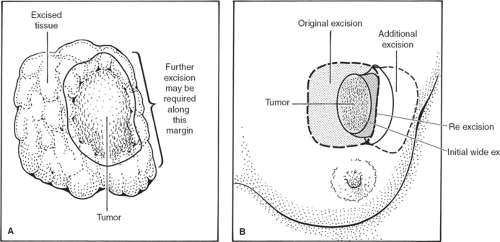
Having lifted the tumor and surrounding breast tissue off the chest wall muscles, the cancer and surrounding tissue are grasped between the finger and the thumb of the nondominant hand and excision is completed at the other margins (Fig. 3). The specimen is immediately orientated prior to submission to the pathologist with sutures, ligaclips, or metal markers (Fig. 4). Using metal markers or ligaclips has the advantage of allowing orientated anteroposterior-intraoperative specimen radiography to be performed. This helps the surgeon to determine that the target lesion has been excised and permits assessment of the completeness of excision at radial margins. If the specimen radiograph shows that the cancer or any associated microcalcification is close to a radial margin, then the surgeon can remove further tissue from the margin of concern, orientate this tissue, and send it for pathologic evaluation (Fig. 5A,B).
Having excised the cancer from the breast, suturing the defect in the breast without mobilization of the breast tissue usually results in distortion of the breast contour. Small defects (<5% breast volume) can be left open and can produce a good final cosmetic result. Larger defects in the breast should be closed by mobilizing the surrounding breast tissue from both the overlying skin and subcutaneous tissue and the underlying chest wall. If large defects (>10% breast volume) are not closed, they fill with seroma, which later absorbs; as scar tissue forms, this contracts, often producing an ugly, distorted breast. The extent to which breast tissue can be mobilized depends on its density and type. Soft fatty breast tissue cannot be mobilized widely as its blood supply is such that tissues at the edges of the excision undergo fat necrosis and produce a very poor final cosmetic result. In contrast, dense fibrous breast tissue can be mobilized widely to fill defects and heals well. Following large-volume excisions, having mobilized breast tissue, it is usually possible to close the defect in the breastplate with a series of interrupted absorbable sutures. Larger defects can be filled by using a latissimus dorsi muscle miniflap, local flaps, or more major breast reshaping as part of a unilateral or bilateral therapeutic mammoplasty. Drains are not necessary following wide local excision and should not be used routinely. They do not protect against hematoma formation and increase infection rates. Breast skin wounds should be closed in layers with absorbable sutures, finishing with a subcuticular suture. Staples and interrupted sutures are not an acceptable method of wound closure in the breast. Local anesthetic, usually a combination of bupivacaine and adrenaline, infiltrated into the wound reduces postoperative pain.
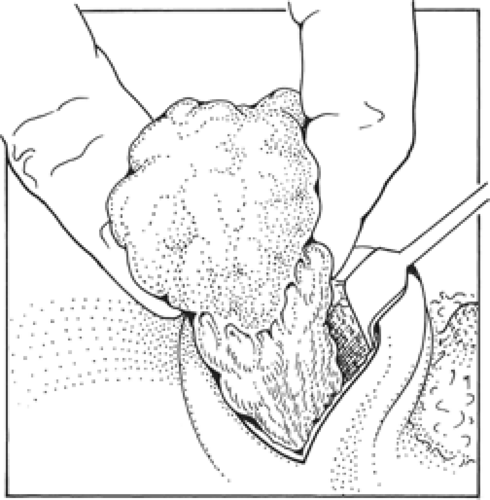 Fig. 3. After having dissected around three sides of the cancer, it is grasped with finger and thumb of the nondominant hand before completing the excision at the other margins. |
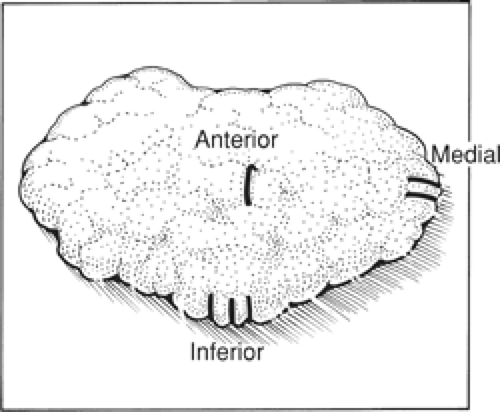 Fig. 4. Orientation of the specimen. In our unit, we use one ligaclip for the anterior margin, two ligaclips for the medial margin, and three ligaclips at the inferior margin. |
Available Techniques for Wide Local Excision of Central Tumors
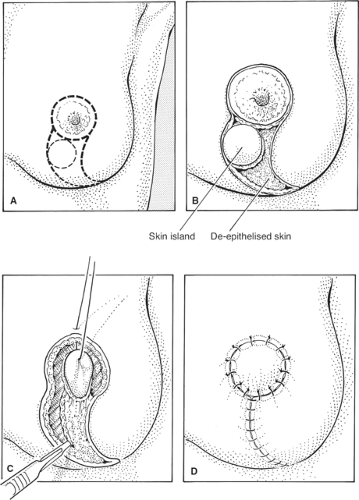
Central cancers can be removed from under the nipple, if they are not very superficial, by a standard wide excision preserving the overlying nipple skin. If the lesion is very superficial and there is tethering, inversion, or invasion of the nipple, then it is usually necessary to remove the nipple and/or areolar complex. In women with large breasts, the nipple–areolar complex can be incorporated into an elliptical incision or a vertical wedge mammaplasty incision and the cancer excised in continuity with the nipple/areolar skin. Although this does alter the breast shape, acceptable results can be obtained in women with larger breasts. Following nipple–areolar excision, the areola can be closed by a purse-string closure. The final cosmetic result is variable but can sometimes produce an excellent long-term cosmetic outcome. An alternative approach for such cancers involves removal of the nipple–areolar complex with an underlying cone of breast tissue down to the pectoral fascia. The breast is then reconstructed with a skin and breast tissue flap rotated in
from the lower outer quadrant of the breast. To do this an island of skin is identified and marked (Fig. 6A). The flap of tissue to be rotated to fill the defect is defined and, apart from the circle of skin that will close the central cutaneous defect, the rest of the skin is deepithelialized (Fig. 6B). The breast tissue is incised and divided (Fig. 6C) so that the flap can rotate and allow the island of skin to lie in the position of the areola (Fig. 6D). The flap is sutured in situ with absorbable sutures.
from the lower outer quadrant of the breast. To do this an island of skin is identified and marked (Fig. 6A). The flap of tissue to be rotated to fill the defect is defined and, apart from the circle of skin that will close the central cutaneous defect, the rest of the skin is deepithelialized (Fig. 6B). The breast tissue is incised and divided (Fig. 6C) so that the flap can rotate and allow the island of skin to lie in the position of the areola (Fig. 6D). The flap is sutured in situ with absorbable sutures.
Technique of Excising Impalpable Cancers
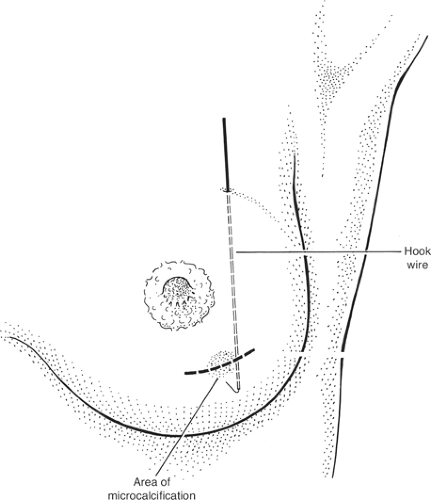
Impalpable lesions can be localized prior to surgery using a number of different techniques, including skin marking, injection of blue dye, carbon, or radioisotope, insertion of a hook wire with postlocalization mammograms (Fig. 7), or use of intraoperative ultrasound by the surgeon. Excising an impalpable cancer is easier if the skin incision is made directly over the cancer. If the lesion is being localized by ultrasound, the skin directly over the cancer should be marked along with the depth of the cancer below the skin by the radiologist performing the localization. If the lesion is only visible on a radiograph, then from the mammograms, it is usually possible for the surgeon to locate the skin directly over the lesion. An appropriate-sized skin incision is made and deepened (Fig. 8). If a wire is in place, dissection continues toward the wire so that it can be located above where it enters the lesion (Fig. 9).
Localization wires with markings, which change diameter or have a guide that can be placed over the wire, help the surgeon know exactly how far down the wire the lesion is situated. Some surgeons use multiple wires to bracket a lesion. This can be helpful but is time-consuming for the radiologist and while valuable in the more difficult widespread calcifications is not always necessary. The direction of the wire on the preoperative mammograms is only a guide to the actual path of the wire through the breast, and can be misleading. The aim is to remove the mammographic lesion with a 1-cm clear radiologic margin (Fig. 10). Radio-guided occult lesion localization is a newer technique for localization of impalpable lesions; it was first introduced at the European Institute of Oncology in Milan. Under mammogram or ultrasound control, technetium-labeled human serum albumin or sulfur colloid is injected into the tumor. The surgeon then uses a handheld gamma-detecting probe intraoperatively to locate the lesion to guide excision. There is some evidence that this technique may be superior to hook wire localization.
The specimen, having been excised, should be orientated with ligaclips or metal markers, or secured to an orientated grid so that an orientated-specimen radiograph can be obtained. Radiographs are obtained following compression in a mammogram machine or noncompressed in an X-ray machine designed to X-ray breast specimens. There have been conflicting reports whether compressing the specimen affects the incidence of subsequent positive margins as reported by the pathologist. Orientated-specimen radiographs improve the rate of complete excision of impalpable cancers. The patient should remain anesthetized until the specimen radiograph has been reviewed by the surgeon and the radiologist and both need to be satisfied that the cancer and all radiologically visible suspicious areas have been removed before the patient is wakened.
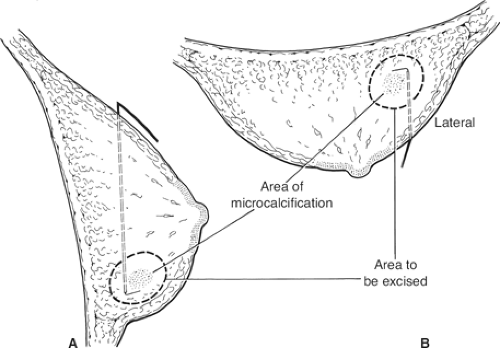 Fig. 7. Diagrammatic representation of mammograms illustrating the position of a hook wire in relation to the area of microcalcification. A: Lateral view. B: Craniocaudal view. |