KEY CONCEPTS
![]() Breast cancer is usually diagnosed in the early stages when it is a highly curable malignancy.
Breast cancer is usually diagnosed in the early stages when it is a highly curable malignancy.
![]() Local therapy of early-stage breast cancer consists of modified radical mastectomy or lumpectomy plus external-beam radiation therapy. The surgical approach to the ipsilateral axilla may consist of a lymph node mapping procedure with sentinel lymph node biopsy or a full level I/II axillary lymph node dissection.
Local therapy of early-stage breast cancer consists of modified radical mastectomy or lumpectomy plus external-beam radiation therapy. The surgical approach to the ipsilateral axilla may consist of a lymph node mapping procedure with sentinel lymph node biopsy or a full level I/II axillary lymph node dissection.
![]() Adjuvant endocrine therapy reduces the rates of relapse and death in patients with hormone receptor–positive early breast cancer. Adjuvant chemotherapy reduces the rates of relapse and death in all patients with early-stage breast cancer.
Adjuvant endocrine therapy reduces the rates of relapse and death in patients with hormone receptor–positive early breast cancer. Adjuvant chemotherapy reduces the rates of relapse and death in all patients with early-stage breast cancer.
![]() The choice of the most appropriate chemotherapy, endocrine therapy, and anti-HER2 regimen is complex and rapidly changing as results from ongoing randomized clinical trials are reported.
The choice of the most appropriate chemotherapy, endocrine therapy, and anti-HER2 regimen is complex and rapidly changing as results from ongoing randomized clinical trials are reported.
![]() Neoadjuvant chemotherapy and biotherapy are appropriate for selected patients with early breast cancer and most patients with locally advanced breast cancer and inflammatory breast cancer followed by local therapy and further adjuvant systemic therapy as indicated.
Neoadjuvant chemotherapy and biotherapy are appropriate for selected patients with early breast cancer and most patients with locally advanced breast cancer and inflammatory breast cancer followed by local therapy and further adjuvant systemic therapy as indicated.
![]() Whereas the goal of adjuvant and neoadjuvant chemotherapy is curative, the goal of chemotherapy in the metastatic setting is palliative.
Whereas the goal of adjuvant and neoadjuvant chemotherapy is curative, the goal of chemotherapy in the metastatic setting is palliative.
![]() Initial therapy of metastatic breast cancer in most women with hormone receptor–positive tumors should include endocrine therapy.
Initial therapy of metastatic breast cancer in most women with hormone receptor–positive tumors should include endocrine therapy.
![]() About 60% of women with metastatic breast cancer will respond to chemotherapy regimens; anthracycline- and taxane-containing regimens are the most active.
About 60% of women with metastatic breast cancer will respond to chemotherapy regimens; anthracycline- and taxane-containing regimens are the most active.
![]() Anti-HER2 therapies and other biologic or targeted agents (e.g., everolimus) in combination with chemotherapy or endocrine therapy have significantly improved outcomes for selected patients with metastatic breast cancer.
Anti-HER2 therapies and other biologic or targeted agents (e.g., everolimus) in combination with chemotherapy or endocrine therapy have significantly improved outcomes for selected patients with metastatic breast cancer.
![]() Although controversial, regular screening mammography in women younger than 50 years of age is beneficial, and many national and international studies demonstrate a reduction in the breast cancer mortality rate from annual or biennial screening mammography in women ages 50 to 74 years.
Although controversial, regular screening mammography in women younger than 50 years of age is beneficial, and many national and international studies demonstrate a reduction in the breast cancer mortality rate from annual or biennial screening mammography in women ages 50 to 74 years.
INTRODUCTION
Breast cancer is the most common site of cancer and is second only to lung cancer as a cause of cancer death in American women. It was estimated that 234,580 new cases of breast cancer will be diagnosed and that 40,030 people will die of breast cancer in 2013.1 In addition to invasive breast cancers, it is estimated that 64,640 cases of noninvasive, or in situ, cancer will be diagnosed among women in the United States in 2013.
Female breast cancer incidence rates vary considerably across racial and ethnic groups. The average annual age-adjusted incidence rate from 2004 to 2008 was 122.3 cases per 100,000 among whites, 116.1 cases among African Americans, 92.3 cases in Hispanics, 89.2 cases in American Indians and Alaska Natives, and 84.9 cases among Asian Americans and Pacific Islanders.2 Reasons for the higher incidence rates in whites than in other racial and ethnic groups may include differences in reproductive and lifestyle factors and access to and use of screening.
Female breast cancer incidence rates have increased for all women combined since 1980, although the rate of increase slowed in the 1990s and has decreased starting in 2000 after peaking in 1999. The decrease in breast cancer incidence of about 7% from 2002 to 2003 is thought to be related to decreased use of postmenopausal hormone replacement therapy (HRT).2 Incidence rates were stable from 2004 to 2008. The incidence of ductal carcinoma in situ (DCIS) also increased rapidly between the early and late 1980s and continues to increase. The increase in DCIS is largely attributed to an increased use of screening mammography because most cases of DCIS manifest solely as clustered microcalcifications seen on mammography.1
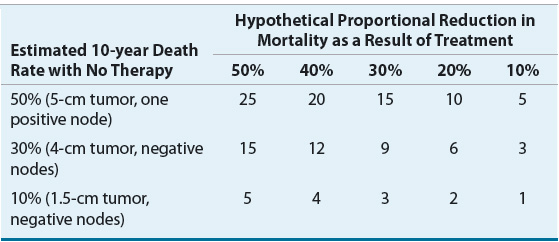
![]() For all racial and ethnic groups, most breast cancers are diagnosed at an early stage when tumors are small and localized. However, a higher proportion of disease is diagnosed at more advanced stages in African American and other minority women than in white women. The death rate is also higher among African American women than white women despite the lower incidence. From 2004 to 2008, the breast cancer death rate was highest in African Americans (32.0 cases per 100,000 women) followed by whites (22.8), American Indians and Alaska Natives (17.2), Hispanics (15.1), and Asian Americans and Pacific Islanders (12.2).2 The cause of this disparity between white and African American women is widely debated and multifactorial, with possible explanations including access to care, socioeconomic status, cultural differences, higher stage at diagnosis, and more aggressive biologic features. Despite these differences, overall mortality rates from breast cancer in the United States have declined since 1990. These declines have been attributed to increased use of screening and effectiveness of adjuvant treatment.3–5 Figure 105-1 shows the temporal trends in incidence and mortality by race.
For all racial and ethnic groups, most breast cancers are diagnosed at an early stage when tumors are small and localized. However, a higher proportion of disease is diagnosed at more advanced stages in African American and other minority women than in white women. The death rate is also higher among African American women than white women despite the lower incidence. From 2004 to 2008, the breast cancer death rate was highest in African Americans (32.0 cases per 100,000 women) followed by whites (22.8), American Indians and Alaska Natives (17.2), Hispanics (15.1), and Asian Americans and Pacific Islanders (12.2).2 The cause of this disparity between white and African American women is widely debated and multifactorial, with possible explanations including access to care, socioeconomic status, cultural differences, higher stage at diagnosis, and more aggressive biologic features. Despite these differences, overall mortality rates from breast cancer in the United States have declined since 1990. These declines have been attributed to increased use of screening and effectiveness of adjuvant treatment.3–5 Figure 105-1 shows the temporal trends in incidence and mortality by race.
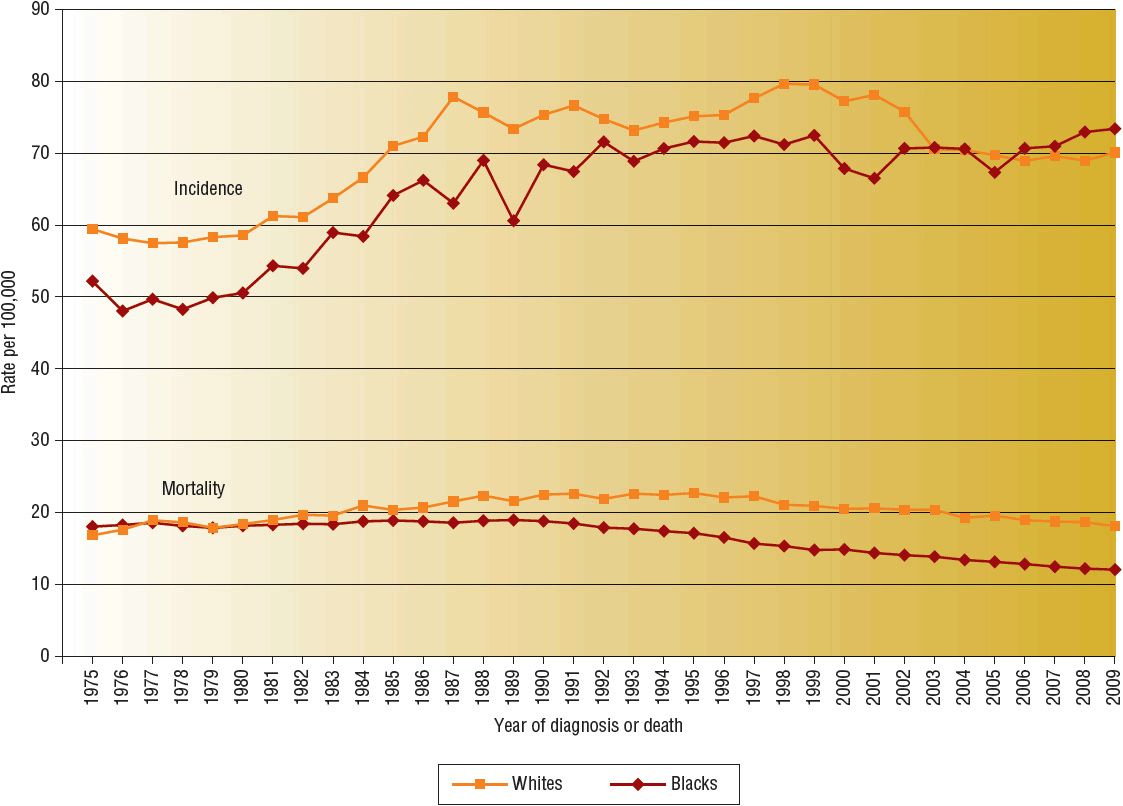
FIGURE 105-1 Breast cancer incidence and mortality rates by race, 1975 to 2009. (Data from Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009 [Vintage 2009 Populations]. Bethesda, MD: National Cancer Institute. 2009, http://seer.cancer.gov/csr/1975_2009_pops09.)
The median age at diagnosis for breast cancer is between the ages of 60 and 65 years.2 Although lung cancer is the leading cause of cancer deaths for women regardless of age, breast cancer is the leading cause of cancer deaths for females between the ages of 20 and 59 years.1
EPIDEMIOLOGY AND ETIOLOGY
The two variables most strongly associated with the occurrence of breast cancer are gender and age. Although one commonly thinks of breast cancer as a disease confined to women, about 2,240 cases of male breast cancer were estimated to be diagnosed in the United States in 2013.1 Male gender had been considered a poor prognostic factor in some investigations, but it is now believed that higher mortality rates in men are attributable to more advanced disease at the time of diagnosis. When stage and other known prognostic factors are controlled for, the clinical outcome for men with breast cancer is comparable to that of women.6 Treatment of breast cancer in men is similar to treatment of breast cancer in women.
The incidence of breast cancer increases with advancing age. A frequently quoted breast cancer statistic is that one in eight women will develop breast cancer during her lifetime. It should be emphasized that this is a cumulative lifetime risk of developing the disease from birth to death. The one-in-eight women figure is often misinterpreted by women who assume that it translates into one in eight women being diagnosed with breast cancer each year. A more useful method of presenting the risk data is based on age intervals.7 Table 105-1 shows that the risk of a woman developing breast cancer before the age of 40 years is about one in 203, and more than half the risk occurs after age 60 years.
TABLE 105-1 Risk of Developing Breast Cancer, Women, All Races, 2006 to 2008
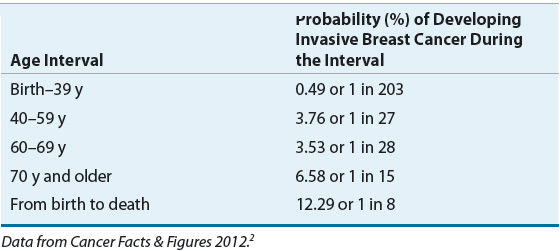
An understanding of the relationship between age and the incidence of breast cancer is particularly relevant when one discusses “risk factors” or factors other than age that increase a woman’s probability of developing breast cancer. The relative risk (RR) of developing breast cancer for an individual woman in a defined risk group is usually multiplied by the probability of a woman developing breast cancer during her lifetime, and this figure is taken as the cumulative lifetime risk of that individual developing breast cancer. However, the risk of developing breast cancer depends on age. Therefore, a more meaningful way to counsel patients regarding their risk of developing breast cancer based on the presence of a known risk factor incorporates an age-specific incidence rate, not cumulative lifetime risk. For example, if a 40-year-old woman with a strong family history of breast cancer has a RR ratio of 2.0, her risk of developing breast cancer by the age of 50 years is only 7.6% (2 × 3.8), not 24.6% (2 × 12.3) (Table 105-1). It is also important to note that recognized risk factors are not additive in a simple mathematical sense. Finally, most women with breast cancer have no identifiable major risk factor, indicating that the search for the etiology of this disease is largely incomplete.
A number of calculators are available to estimate a patient’s risk of developing breast cancer. The National Cancer Institute has an online version of the Breast Cancer Risk Assessment Tool (www.cancer.gov/bcrisktool/Default.aspx). This tool is based on a statistical model known as the Gail model, derived from data from the Breast Cancer Detection and Demonstration Project, a mammography screening project conducted in the 1970s. The Breast Cancer Risk Assessment Tool was designed for healthcare professionals to project a woman’s individualized risk for invasive breast cancer over a 5-year period and over her lifetime. This model has been shown to provide accurate estimates in white women, but it has not been validated for other racial and ethnic groups and other subgroups, including those with genetic risk factors. Other risk assessment models also exist, each taking into account different risk factors. Gail and colleagues have developed a similar model for assessing the risk of developing breast cancer in African American women.8 These empiric models may not be as useful for women with a history suggestive of hereditary breast cancer. Thus, no one model is appropriate for every patient.
Endocrine Factors
A number of endocrine factors have been linked to the incidence of breast cancer.9,10 Many of these relate to the total duration of menstrual life. Early menarche, generally defined as menstruation beginning before age 12 years, increases the cumulative lifetime risk of breast cancer development. Similarly, a late age of natural menopause (age 55 years or later) increases the risk of breast cancer development, although to a lesser degree than early menarche.9 Conversely, bilateral oophorectomy before age 40 years reduces the risk of developing breast cancer.
Nulliparity and a late age at first birth (≥30 years) are reported to increase the lifetime risk of developing breast cancer. It is suggested that the period between the onset of menses and the age of first pregnancy provides a “window of initiation” for the development of breast cancer. This is a time when an unbalanced hormonal environment reacts with the abundant and highly responsive breast tissue. Investigators postulate that international differences in age of menarche, age at menopause, and childbearing may account for a substantial part of the international differences in the incidence of breast cancer.
Many studies have evaluated the relationship between exogenous hormones and the development of breast cancer. Postmenopausal estrogen replacement therapy has been the subject of several epidemiologic studies and meta-analyses, with conflicting results. The National Cancer Institute (NCI)–funded Women’s Health Initiative (WHI) is a series of clinical trials designed to investigate the risks and benefits of treatment strategies that could affect women’s health issues, such as breast cancer. The estrogen plus progestin trial randomized more than 16,000 postmenopausal women to take conjugated equine estrogen combined with medroxyprogesterone or a placebo.11 This study reported an increased risk of breast cancer (38 vs. 30 cases per 10,000 person-years; RR ratio = 1.26; 95%; confidence interval [CI], 1.00–1.59) in women taking combined estrogen and progestin for an average of 5.2 years compared with those receiving placebo. Analysis of the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registries showed that the age-adjusted incidence rate of breast cancer in women in the United States in 2003 fell by 6.7% compared with 2002.12 This decrease in breast cancer incidence seems to be temporally associated with the first report of the WHI study and subsequent decrease in estrogen and progestin HRT use among postmenopausal women. Additional follow-up of patients in this trial confirms a decrease in breast cancer incidence after cessation of estrogen and progestin.13 In the estrogen alone trial, more than 10,000 women who had a hysterectomy and therefore did not require progestin therapy because of a decreased risk of endometrial carcinoma were randomized to estrogen alone or placebo.14 The risk of breast cancer was not increased in women who received estrogen alone compared with those who received placebo. With additional follow-up, the incidence of breast cancer in women in this study was actually lower in patients who received estrogen compared with those who received placebo.15 However, the authors concluded that estrogen alone may not reduce the incidence of breast cancer in patients at increased risk and therefore should not be used specifically for breast cancer risk reduction. Unresolved issues remain as to whether lower doses or short-term use of estrogen or estrogen–progestin for menopausal symptoms can be safe and effective. A longer duration of HRT and concurrent use of progestins appear to contribute to breast cancer risk. The use of postmenopausal HRT in women with a history of breast cancer is generally contraindicated. Women who are considering HRT should carefully consider the risks versus benefits (see Chap. 65) for a detailed discussion of HRT).
Epidemiologic studies of oral contraceptives do not show a consistent relationship between use of birth control pills and breast cancer risk. Results are conflicting, and assessment of the studies should consider the particular oral contraceptive products involved, daily and cumulative doses of the hormones administered, and latency period for development of breast cancer. A meta-analysis of 13 prospective cohort studies conducted between the years of 1989 and 2010 reported a nonsignificant increase in breast cancer incidence for patients who used oral contraceptives compared with those who had never used oral contraceptives.16 Newer formulations of oral contraceptives contain lower hormone concentrations, and the authors of this meta-analysis were not able to differentiate breast cancer risk based on the formulations of oral contraceptives. It is also important to note that oral contraceptives are known to reduce the risk of ovarian and endometrial cancers. Most experts believe that the safety and benefits of low-dose oral contraceptives currently outweigh the potential risks.
Genetic Factors
Both personal and family histories influence a woman’s risk of developing breast cancer. A personal history of breast cancer is associated with an increased risk of developing contralateral breast cancer. Cancers of the uterus and ovary are also associated with an increased risk of developing breast cancer. A number of cancer family syndromes include breast cancer in association with other types of cancers.
Many women have “lumpy breasts” or have a clinical diagnosis of fibrocystic breast disease or benign breast disease. Nonproliferative lesions, such as cysts or simple fibroadenomas, do not increase the risk of breast cancer. Proliferative lesions without atypia, such as intraductal papillomatosis, are associated with a mildly elevated breast cancer risk of about 1.5 to 2.0 times that of the general population. Atypical hyperplasias are classified as either ductal or lobular units, and these lesions may increase a woman’s risk for breast cancer to about 4.5 to 5.0 times that of the general population.17
Dense breast tissue reduces the sensitivity of mammography in detecting breast cancer and is associated with an increased risk of breast cancer. The risk of breast cancer in women with dense breasts (defined by mammography) has been estimated to be between two and six times that of women of the same age with little density.18 Many variables, including age, weight, menopausal status, HRT, and parity, can influence mammographic breast density. Genetic factors may also play a role in this finding because mammographic breast density has been shown to have high heritability and is also strongly associated with a positive family history of breast cancer.
The percentage of all breast cancers in the U.S. population that can be attributed to family history is about 10%. Empirical estimates of the risks associated with particular patterns of family history of breast cancer indicate the following19:
1. Having any first-degree relative with breast cancer increases a woman’s risk of breast cancer about 1.5- to 3-fold. Risk increases with increasing numbers of affected first-degree relatives.
2. The risk is affected by both a woman’s own age and the age of the relative when diagnosed. A higher risk is seen when a woman and her relative at diagnosis are younger than 50 years.
3. The risk associated with having any second-degree relative with breast cancer is complex and depends on other family history patterns. However, the risk is generally lower than that of first-degree relatives.
4. Affected family members on both the maternal and the paternal sides are important to consider in evaluation of risk.
Although women with a family history of breast cancer are at increased risk for the disease, the diagnosis of breast cancer is still uncommon in young women even with a positive family history.
Germ-line mutations in either BRCA1 or BRCA2 are associated with an increased risk for breast and ovarian cancer. These genes function as tumor suppressor genes, maintaining genomic integrity and DNA repair. Compared with an average woman’s 13% lifetime risk of developing breast cancer, the probability of developing breast or ovarian cancer by the age of 70 years in women with a BRCA1 or BRCA2 mutation is estimated to be 57% and 49% for breast cancer and 40% and 18% for ovarian cancer, respectively.20
The probability of being a BRCA gene mutation carrier is related to ethnicity and family history. Jewish people of Eastern European decent (Ashkenazi Jews) have an unusually high (2.5%) carrier rate of germ-line mutations in BRCA1 and BRCA2 compared with the rest of the U.S. population. Conversely, it is estimated that clinically significant BRCA mutations occur at a frequency of about one in 500 persons in the general, non-Jewish U.S. population.21 Testing for BRCA1 and BRCA2 mutations is now widely available, but testing is generally recommended only when there is personal or family history suggestive of hereditary cancer, when the test results can be adequately interpreted, and when results will assist with diagnosis and management. The decision to test an individual for a genetic mutation related to breast cancer risk is complex, and several organizations have published recommendations on genetic susceptibility testing for individuals who meet the criteria for increased risk.22–25
Although most genetic causes of breast cancer are attributed to BRCA1 and BRCA2, other genes that have been identified as being associated with hereditary breast cancer include TP53, CHK2, PTEN, ATM, and others.26
Environmental and Lifestyle Factors
Breast cancer incidence rates vary considerably among countries, which suggests that environmental and lifestyle factors play an important role in the etiology. Compelling evidence is derived from studies of Asian women who migrated to the United States. Although the incidence of breast cancer in Asian women is quite low, the incidence of breast cancer in Asian women who were born in the United States or who migrated from Asia to the United States gradually increases over the individual’s lifetime to equal that of the white population in the same geographic area.27
Diet is an important and modifiable environmental risk factor. Possible relationships between fat intake and steroid hormone metabolism have led to an emphasis on dietary fat as a possible etiologic agent for breast cancer. Epidemiologic data show a positive correlation between higher dietary fat intake and breast cancer risk, which is stronger in postmenopausal than in premenopausal women. In a meta-analysis of 31 case-control and 14 cohort studies on dietary fat and breast cancer, Boyd et al. reported a small but significant RR ratio of 1.13 (95% CI, 1.03–1.25) when comparing the highest and lowest fat intake categories.28 To confirm this association prospectively, the hypothesis that low dietary fat intake reduces breast cancer risk was further tested in the WHI Randomized Controlled Dietary Modification Trial.29 More than 48,000 postmenopausal women were randomized to a dietary intervention that consisted of reducing total fat intake to 20% of energy and consuming at least five servings of fruits and vegetables daily and six servings of grains daily versus a comparison group without any dietary interventions. Over an 8-year mean follow-up period, the incidence of invasive breast cancer was not significantly different between the two groups (annualized incidence rate, 0.42% vs. 0.45%; hazard ratio [HR], 0.91; 95% CI, 0.83–1.01). Although there is still much to be learned about the effects of diet on the risk of developing breast cancer, a low-fat diet seems to be a reasonable approach to potentially reduce the risk of breast cancer.
An additional dietary factor to be explored in the breast cancer population includes food-derived heterocyclic amines, which are known carcinogens found commonly in cooked red meat or processed meat. Studies of red or processed meat ingestion and breast cancer incidence are inconsistent, and no association was reported in one meta-analysis.30
Many studies have also examined the association between breast cancer and intake of dietary fiber and micronutrients, including β-carotene, and vitamins A, C, and E. The relationship between vitamins and breast cancer is unclear. No consistent benefit of fruits or vegetable consumption and the risk of breast cancer has been demonstrated.30
Another dietary factor that deserves mention is the possible effect of phytoestrogens on breast cancer risk. Phytoestrogens are natural plant estrogens found in soybean products, seeds, berries, and nuts. The two most studied classes of dietary phytoestrogens are isoflavones and lignans; isoflavones are richer in Asian diets, and lignans are the main source of phytoestrogens in the Western diet.31,32 Because these compounds exhibit weak estrogenic properties, some experts believe that they may function as relative antiestrogens by displacing natural estradiol. However, studies have also reported a potential stimulatory effect on breast tissue. A meta-analysis of observational studies that evaluated phytoestrogen use and the risk of breast cancer suggests that any potential associated risk reduction is modest and may be limited to postmenopausal patients.31 Nonetheless, the effect of phytoestrogens on breast cancer is very controversial, and further research is needed.
Both body weight and height are associated with the incidence of breast cancer. Most studies of premenopausal women show either no relationship with body weight or slightly declining breast cancer risks with increasing body weight. Most studies in postmenopausal women show increasing breast cancer risks with increasing body weight. Accordingly, a meta-analysis by Renehan et al. found that an increase in body mass index was associated with an increase in the risk of breast cancer for postmenopausal women (RR, 1.12; 95% CI, 1.08–1.16; P <0.0001) but had the opposite effect in premenopausal women (RR, 0.92; 95% CI, 0.88–0.97; P <0.001).33 An increase in circulating estrogen is postulated to be the most likely explanation for these results. Although height is not a modifiable risk factor, weight and body composition are modifiable and should be studied further. Maintaining a healthy weight and body composition appear to be beneficial and promote many different health benefits but requires further study in association with the incidence of breast cancer.
Many studies report an inverse association between physical activity and breast cancer risk.34 A review of 19 cohort and 29 case-control studies suggests that the association is stronger for postmenopausal breast cancer than for premenopausal breast cancer. Exercise may provide modest protection against breast cancer, but the relationship is complex. Possible explanations include the effects of physical activity on menstrual characteristics (in premenopausal women), body size, weight, and serum hormone levels. Estrogen-related pathways or other metabolic hormones such as insulin and insulin-like growth factors may influence this relationship. Making healthy choices appears to be the best health advice for women.
Many epidemiologic studies have evaluated the relationship between alcohol and breast cancer. Studies indicate both a modest positive association between alcohol and breast cancer and a dose–response relationship.35 The risk increases with consumption of alcohol in general regardless of the beverage type or woman’s menopausal status. Although the exact mechanism is unknown, the most plausible biologic hypothesis relates to increased levels of estrogen or other reproductive steroid hormones caused by impaired liver function. Although a causal relationship between alcohol consumption and breast cancer has not been proven in a prospective trial, the weight of the available evidence suggests that a relationship (direct or indirect) may exist. Because alcohol consumption is a modifiable risk factor, use in moderation appears to be a sensible approach.
Radiation to the breast tissue is associated with an increased risk of breast cancer, particularly with exposure at a young age (<20 years), again suggesting that a “window of initiation” for breast cancer occurs at a relatively early age. Much of the knowledge about radiation-related breast cancer comes from epidemiologic studies of patients exposed to diagnostic or therapeutic radiation and of Japanese survivors of the atomic bombs.36 Women treated with chest irradiation for Hodgkin lymphoma in childhood or adolescence and survivors of other childhood cancers (in which radiation is used as a mainstay of therapy) are among the populations at greater risk for secondary breast cancers. The risk increases linearly with radiation dose. Exposure to diagnostic x-rays, including annual screening mammography, does not impart a sufficient dose of radiation for clinical concern in the general population. However, the risk of breast cancer after radiation exposure even in low levels in those with genetic risk factors is unclear and is an ongoing area of research.
In conclusion, numerous studies have been performed to investigate potential causative factors in the etiology of breast cancer. Several endocrine, genetic, environmental, and lifestyle factors are associated with the development of breast cancer to varying degrees. Some factors are modifiable, but others are not. Additionally, the impact of individual risk factors may vary depending on other confounding variables such as age, family history, estrogen use, and menopausal status. Although epidemiologic studies provide a large body of the current evidence, they have their limitations, and results are varied. Meta-analyses summarize numerous study results, but heterogeneity of studies may limit the applicability of the evidence. Additional prospective, randomized controlled trials are needed to confirm the importance of factors that are associated with the risk of developing breast cancer.
CLINICAL PRESENTATION
A painless lump is the initial sign of breast cancer in most women. The typical malignant mass is solitary, unilateral, solid, hard, irregular, and nonmobile. In small numbers of cases, stabbing or aching pain is the first symptom. Less commonly, nipple discharge, retraction, or dimpling may herald the onset of the disease. In more advanced cases, prominent skin edema, redness, warmth, and induration of the underlying tissue may be observed.
The breast is a complex organ composed of skin, subcutaneous tissue, fatty tissue, and branching ductal and glandular structures (Fig. 105-2). Various diseases that affect these structures can produce a palpable mass. In addition, the physiologic changes associated with the menstrual cycle can cause normal breast changes. Common causes of breast masses in young women are fibroadenoma, fibrocystic disease, carcinoma, and fat necrosis.
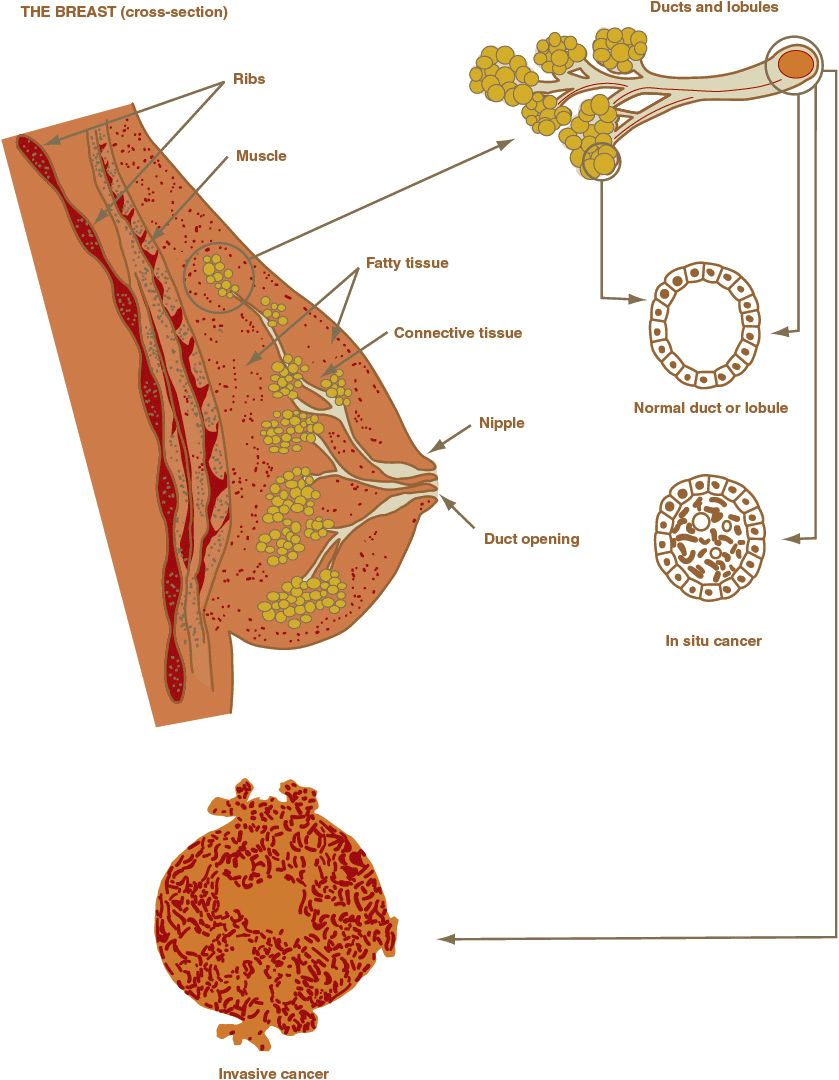
FIGURE 105-2 Breast anatomy.
Many women detect some breast abnormality themselves, but in the United States, it is increasingly common for breast cancer to be detected during routine screening mammography in asymptomatic women. It is widely accepted that the smaller the mass, the higher the likelihood of cure. Thus, as the number of breast cancer cases found by screening mammography increases, overall survival (OS) of breast cancer patients has improved; however, this decreasing mortality rate is also related to improved systemic therapy.
CLINICAL PRESENTATION
Breast cancer that is confined to a localized breast lesion is often referred to as early, primary, localized, or curable. Breast cancer that has spread to local–regional lymph nodes is still considered early stage (Fig. 105-3). Unfortunately, breast cancer cells often spread by contiguity, through lymph channels, and through the blood to distant sites. This often occurs early in breast cancer growth, and deposits of tumor cells form in distant sites that are undetected with current diagnostic methods and equipment (micro-metastases). When breast cancer cells can be detected clinically or radiologically in sites distant from the breast, the disease is referred to as advanced or metastatic breast cancer. Tissues most commonly involved with distant metastases are lymph nodes (other than local–regional lymph nodes), skin, bone, liver, lungs, and brain. Symptoms of bone pain, difficulty breathing, abdominal enlargement, jaundice, and mental status changes may herald the clinical presentation of metastatic breast cancer. A small percentage of women have signs and symptoms of distant metastases when they first seek treatment. In virtually all of them, a neglected breast mass has been present for several months to years. In addition, about half of all patients who initially are treated for localized disease eventually develop signs and symptoms of metastatic breast cancer.
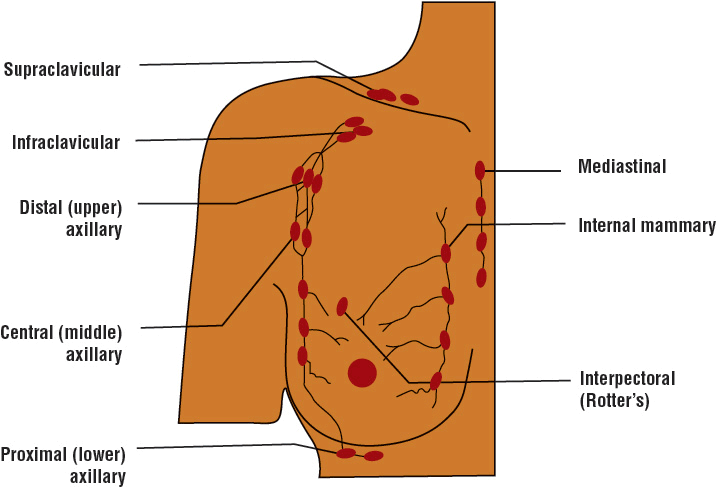
FIGURE 105-3 Lymph node anatomy.
DIAGNOSIS
The initial workup for a woman presenting with a breast mass or symptoms suggestive of breast cancer should include a careful history, physical examination of the breast, three-dimensional mammography, and possibly other breast imaging techniques such as ultrasonography or magnetic resonance imaging (MRI). Most breast cancers can be visualized on a mammogram as a mass, a cluster of calcifications, or a combination of these findings. Specific mammographic features associated with the highest risk of malignancy include masses with spiculated margins or an irregular shape and calcifications with a linear or segmental distribution.37 One major factor that affects the ability of mammography to detect cancer includes breast density (the fat-to-glandular tissue ratio of the breast), which may be affected by age, menopausal status, and HRT use. Ultrasonography, MRI, and digital mammography are alternate breast imaging methods that are being investigated for women with dense breasts or other specific subsets of patients with breast cancer (e.g., MRI in patients with inflammatory breast cancer).38 The technical quality of the examination and the expertise of the radiologist are also important factors.
The Breast Imaging Reporting and Data System (BI-RADS) was developed by the American College of Radiology to standardize mammographic reporting.39 There are seven assessment categories (0–6) with four possible recommendations: (a) additional imaging evaluation, (b) routine interval screening, (c) short-term follow-up, and (d) biopsy. The probability of a biopsy positive for malignancy increases from less than 2% for BI-RADS category 3 mammograms to 20% to 30% for category 4 mammograms, to greater than 95% for category 5 mammograms. Similar categories for reporting have also been developed for breast MRI and ultrasonography.
Breast biopsy is indicated for a mammographic abnormality that suggests malignancy or for a palpable mass on physical examination. Three techniques are available: fine-needle aspiration, core-needle biopsy, and excisional biopsy.40 Excisional biopsy completely removes the abnormal tissue. Needle biopsies are performed percutaneously and include both core-needle biopsy (which removes a core of tissue) and fine-needle aspiration (which removes cells from the suspicious site). Core-needle biopsy is the preferred biopsy method for mammographically detected, nonpalpable abnormalities.38 Core-needle biopsy offers a more definitive histologic diagnosis, avoids inadequate samples, and can distinguish invasive from in situ breast cancer (which fine needle biopsy cannot). After confirmation of malignancy via core-needle biopsy, subsequent surgical procedures are performed (either before or after systemic therapy) to assure complete removal of the abnormal tissue.
STAGING AND PROGNOSIS
Breast cancer stage is defined on the basis of the primary tumor extent and size (T1–4), presence and extent of lymph node involvement (N1–3), and presence or absence of distant metastases (M0–1) (Table 105-2 and Fig. 105-4). Although many possible combinations of T and N are possible within a given stage, simplistically, stage 0 represents carcinoma in situ (Tis) or disease that has not invaded the basement membrane of the breast tissue. Stage I represents a small primary invasive tumor without lymph node involvement or with micrometastatic nodal involvement, and stage II disease usually involves regional lymph nodes. Stages I and II are often referred to as early breast cancer. It is in these early stages that the disease is highly curable (99% 5-year survival in patients with disease confined to the breast, node negative). Stage III, also referred to as locally advanced disease, usually represents a large tumor with extensive nodal involvement in which either node or tumor is fixed to the chest wall. Stage IV disease is characterized by the presence of metastases to organs distant from the primary tumor and is often referred to as advanced or metastatic disease as described earlier (23% 5-year survival rate in patients with distant metastases). Most breast cancer today presents in early stages where the prognosis is favorable (93% of newly diagnosed patients have disease confined to the breast or local lymph nodes).2
TABLE 105-2 Tumor, Node, Metastasis Stage Grouping for Breast Cancer
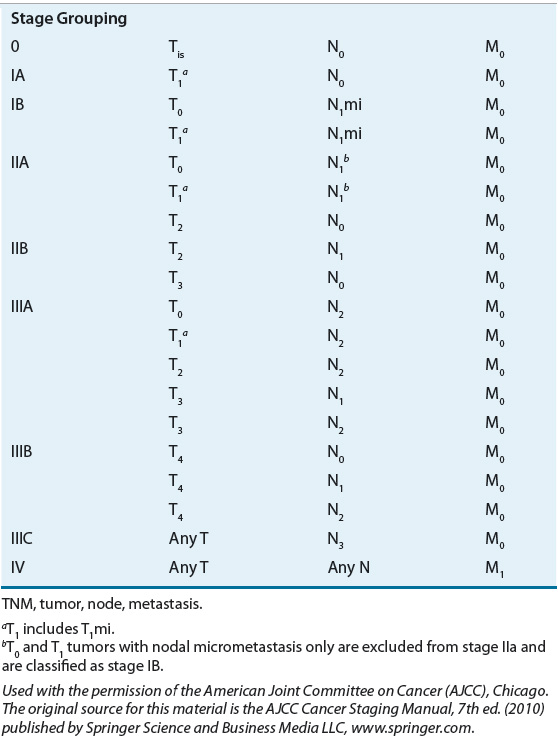
FIGURE 105-4 TNM (tumor, node, metastasis) staging system for breast cancer. (Used with the permission of the American Joint Committee on Cancer [AJCC], Chicago, Illinois. The original source for this material is the AJCC Cancer Staging Manual, 7th ed. [2010] published by Springer Science and Business Media LLC, www.springer.com.)
Staging for breast cancer is separated into two groups, clinical and pathologic. Clinical staging is assigned before surgery and is based on physical examination (assessment of tumor size and presence of axillary lymph nodes), imaging (mammography, ultrasonography, and so on), and pathologic examination of tissues (e.g., biopsy results). Pathologic staging occurs after surgery and uses information from clinical staging but adds data from surgical exploration and resection, such as tumor size at surgery and the involvement of micro- or macro-invasive tumor in the lymph nodes or other metastatic sites. Because of the advent of sentinel lymph node biopsy (SLNB; see the Treatment of Early Breast Cancer section), the assessment of lymph node status has become more complex. The American Joint Committee for Cancer (AJCC) publishes staging criteria for cancers, and the breast cancer criteria were most recently updated in January 2010.41 This staging system is widely accepted and used in all breast cancer patients to determine prognosis and assist with treatment decisions. It is also used to report and track breast cancer diagnoses in tumor registries and databases.
PATHOLOGY
The pathologic evaluation of breast tissue serves to establish the histologic diagnosis and to confirm the presence or absence of other factors believed to influence prognosis.
Invasive Carcinoma
Invasive breast cancers are a histologically heterogeneous group of lesions. Most breast cancers are adenocarcinomas and are classified on the basis of their microscopic appearance as ductal or lobular, corresponding to the ducts and lobules of the normal breast (Fig. 105-2). The various histologic types of breast cancer have different prognoses, but it is unknown whether their response to therapy differs because patients in therapeutic trials are not typically stratified according to histologic type. The five most common types of invasive breast cancer are briefly described.42
Invasive or infiltrating ductal carcinoma is the most common histology, accounting for about 75% of all invasive breast cancers. These tumors commonly spread to the axillary lymph nodes, and their prognosis is poorer than for other histologic types (specifically tubular, medullary, and mucinous). Invasive or infiltrating lobular carcinoma accounts for 5% to 10% of breast tumors. Both clinical and radiologic findings for these tumors may be quite subtle. The typical presentation is an area of ill-defined thickening in the breast in contrast to a prominent lump characteristic of infiltrating ductal carcinoma. Infiltrating lobular carcinoma can also be more difficult to detect by mammography. Overall, infiltrating lobular carcinoma and infiltrating ductal carcinoma have similar likelihoods of axillary node involvement and disease recurrence and death, yet the sites of metastases may differ. Whereas Infiltrating ductal carcinoma more frequently metastasizes to the bone or to the liver, lung, or brain, infiltrating lobular carcinoma tends to metastasize to the leptomeninges, peritoneal surfaces, retroperitoneum, gastrointestinal tract, reproductive organs, and other unusual sites.
The three most common special types of invasive cancer are medullary, mucinous, and tubular. The prognosis may be more favorable with these unusual histologies. Medullary carcinoma accounts for fewer than 7% of all breast carcinomas, mucinous (or colloid) carcinoma constitutes about 3%, and tubular carcinoma accounts for about 2% of all breast cancers. Histologies rarely reported include adenocystic carcinoma, carcinosarcomas, metaplastic, cribriform, and papillary carcinoma.
Special situations seen clinically and histologically include Paget’s disease of the breast, phyllodes tumors, and inflammatory breast cancer. Paget’s disease of the breast occurs in 1% to 4% of all patients with breast cancer and is characterized by neoplastic cells in the nipple areolar complex. The patient presents clinically with eczematous changes in the nipple with itching, burning, oozing, bleeding, or some combination of these. In most cases, the nipple changes are associated with an underlying carcinoma in the breast that is usually palpable.
Phyllodes tumors of the breast (also known as cystosarcoma phyllodes) are rare tumors with subtypes that range from benign to malignant. These tumors often enlarge rapidly, are painless, and can appear as fibroadenomas.43
Inflammatory breast cancer is characterized clinically by prominent skin edema, redness and warmth, and induration of the underlying tissue. Biopsies of the involved skin reveal cancer cells in the dermal lymphatics. Inflammatory breast cancer typically has a very rapid onset and is often mistaken for an infectious cellulitis or mastitis. Although it may look somewhat similar to a neglected mass, its presentation with rapid onset and progression of local symptoms distinguishes it from other cases of locally advanced breast cancer. The prognosis of patients with inflammatory breast cancer is poor even if the disease is apparently localized.44
Noninvasive Carcinoma
As with invasive carcinoma, the noninvasive lesions may be divided broadly into ductal and lobular categories. Evidence supports that the development of malignancy is a multistep process and that invasive breast cancer has a preinvasive, in situ phase. During the carcinoma in situ phase, normal epithelial cells undergo genetic alterations that result in malignant transformation. Transformed epithelial cells proliferate and pile up within lobules or ducts but lack the required genetic alterations that enable the cells to penetrate the basement membrane. Therefore, carcinoma in situ is diagnosed when malignant transformation of cells has occurred but the basement membrane is intact.
The widespread use of screening mammography with subsequent biopsy and greater recognition of noninvasive breast carcinoma by pathologists has resulted in a significant increase in the diagnosis of in situ breast cancer over the past decade. Assuming a consistent incidence and survival rates, researchers estimate that the prevalence of noninvasive (in situ) breast will exceed 1 million cases by 2016.45 The natural history of these disorders is not well described, and thus the debate continues regarding carcinoma in situ: Is carcinoma in situ preinvasive cancer or simply a marker of unstable epithelium that represents an increased risk for the development of subsequent aggressive cancer?46,47 Answering this question may change the way noninvasive breast cancers are treated.
Ductal carcinoma in situ is more frequently diagnosed than lobular carcinoma in situ (LCIS). Most cases of DCIS today are found by biopsies performed for clustered microcalcifications seen on screening mammography, a hallmark of this disorder.
The ultimate goal of treatment for noninvasive carcinomas is to prevent the development of invasive disease. If left untreated, it is estimated that 14% to 50% of DCIS lesions will progress to invasive breast cancer.46 Therefore, up to 50% of these tumors do not progress to invasive disease, but identifying this group of patients is not yet feasible, and all diagnoses should be treated. Locoregional treatment of DCIS depends on its location, size, and pathology.48 Treatment options include (a) local excision alone with negative margins, (b) local excision (with negative margins) followed by breast irradiation, and (c) traditional total mastectomy with or without reconstruction. Whole-breast irradiation is recommended after excision to significantly decrease the risk of local recurrence, although there is no evidence that survival differs between the previously mentioned options.48 Excision with negative margins alone without radiation may be considered in patients with small and low-grade DCIS. Mastectomy had been the standard treatment of DCIS for several decades, but long-term survival appears to be equivalent with mastectomy versus local excision and irradiation, and the latter option allows for breast conservation. If more than one area of the breast is involved with DCIS, a mastectomy is the preferred option. Axillary lymph node dissection (ALND) is generally not indicated, although sentinel lymph node biopsy (see the Early Breast Cancer section) may be considered in selected patients.48 Cytotoxic chemotherapy has no role in the treatment of patients with pure DCIS. It is important to determine hormone receptor status on the cancer cells. Tamoxifen treatment for 5 years may be considered in some women with hormone receptor–positive DCIS. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-24 trial, which randomized women with DCIS to lumpectomy with radiation plus either tamoxifen or placebo, showed a benefit with tamoxifen in reducing ipsilateral breast cancer recurrence (44% reduction; P = 0.03).49 Further subgroup analyses of this trial suggest a benefit for patients with estrogen receptor (ER)–positive DCIS. Ongoing clinical trials are evaluating the role of aromatase inhibitors (AIs) in the treatment of postmenopausal hormone receptor–positive DCIS. These treatment decisions are often difficult to discuss with patients because these treatments have toxicities that are worrisome to most patients. Nonetheless, an open and honest conversation regarding the risks and benefits is warranted.
Lobular carcinoma in situ is a microscopic diagnosis. In these cases, there is generally no palpable mass, and no specific clinical abnormality is noted. Unlike DCIS, LCIS does not generally demonstrate calcifications on mammography and in fact is usually undetectable by mammography. Consequently, the diagnosis of LCIS is usually an incidental finding in biopsy specimens obtained because of symptoms or mammography findings consistent with benign lesions. It is unclear whether LCIS is a precursor lesion to invasive carcinoma or serves as a marker of risk for invasive carcinoma developing somewhere in the breast. The risk for developing invasive carcinoma is about 0.5% to 1% per year, and both invasive ductal carcinoma and invasive lobular carcinoma can occur. In about 30% to 50% of patients, there are multiple foci of LCIS in the ipsilateral breast, and the contralateral breast is also affected. Thus, the risk for the development of breast cancer is equally high in either breast, which makes the management of LCIS very controversial.47 Some experts favor a program of observation, with semiannual physical examination and annual mammography.48 In selected patients with high-risk genetic mutations or strong family history and in women who are particularly anxious about the development of cancer, bilateral mastectomies with or without reconstruction may be considered.50 Radiation and systemic chemotherapy have no role in the management of LCIS. The use of chemoprevention with tamoxifen in premenopausal women or tamoxifen, raloxifene, or exemestane in postmenopausal women may also be considered for risk reduction in these patients.50 See Prevention and Early Detection later for details.51–53
PROGNOSTIC FACTORS
The natural history of breast cancer varies among patients, with some having extremely aggressive disease that progresses rapidly and others following a more indolent course. The ability to predict prognosis is extremely important in designing treatment recommendations to maximize quantity and quality of life. A number of pathologic prognostic and predictive factors have been identified. Prognostic factors are characteristics or measurements available at diagnosis or time of surgery that in the absence of adjuvant systemic therapy are associated with recurrence rate, death rate, or other clinical outcomes. Predictive factors are measurements available at diagnosis that are associated with response to a specific therapy. Prognostic and predictive factors fall into three general categories: (a) patient characteristics that are independent of the disease such as age; (b) cancer characteristics such as tumor size or histologic type; and (c) other biomarkers that are measurable parameters in tissues, cells, or fluids, such as hormone receptor status. Ideally, the use of prognostic and predictive factors can limit a specific treatment to patients who are most likely to derive benefit, thus sparing unwanted toxicities in those who are unlikely to benefit.
Age at diagnosis and ethnicity are patient characteristics that may affect prognosis. Some younger patients, particularly those younger than 35 years of age, have more aggressive forms of breast cancer and a worse prognosis. Younger patients are more likely to present with poor prognostic features, such as affected lymph nodes, large tumor size, and tumors negative for hormone receptors. Race and ethnicity may also play a role in breast cancer prognosis. African American women have decreased survival periods compared with white women. The cause of this racial disparity is widely debated, with possible explanations including access to care, socioeconomic status, cultural differences, higher stage at diagnosis, and more aggressive biologic features.
Potentially modifiable prognostic factors include alcohol use, dietary factors, weight, and exercise. The association between breast cancer prognosis and alcohol consumption is not as strong as with alcohol and breast cancer risk. A review of seven observational studies showed that postdiagnosis alcohol consumption was not associated with breast cancer outcomes.54 Two randomized controlled studies examined the effects of diet on the risk of breast cancer with conflicting results, primarily focusing on lowering dietary fat.55,56 One study found an improvement in disease-free survival (DFS) with incorporation of a low-fat diet (less than 15% dietary fat per day versus no intervention),56 but another study found no difference in recurrence rates between two dietary intervention approaches (both incorporating a low-fat, high-fiber approach).55 Although these studies asked different questions and had many confounding variables that potentially affected the results, most clinicians recommend that breast cancer survivors eat a low-fat, high-fiber diet and maintain a healthy weight. Obesity at the time of a breast cancer diagnosis has been shown to increase the risk of breast cancer–specific and overall mortality compared with nonobese breast cancer patients, although the impact of weight loss in this population is unclear.54 Observational studies have reported that exercise in women after a diagnosis of breast cancer may also decrease the likelihood of breast cancer recurrence and breast cancer–related death.54 Based on these data, agencies such as the American Cancer Society (ACS) have recognized that physical activity, weight control, and diet are potentially modifiable risk factors for reducing the risk of recurrent breast cancer and other comorbidities (e.g., heart disease, diabetes).57
Disease characteristics that have been shown to provide important prognostic information include lymph node status, tumor size, histologic subtype, nuclear or histologic grade, lymphatic and vascular invasion, and proliferation indices.
Tumor size and the presence and number of involved lymph nodes are established primary factors in assessing the risk for breast cancer recurrence and subsequent metastatic disease. Table 105-3 shows 5-year survival rates according to size of the primary tumor and axillary node involvement. The major factor that influences the likelihood of recurrence is the presence of positive lymph nodes. However, regardless of lymph node status, the size of the primary tumor remains an independent prognostic factor for disease recurrence.
TABLE 105-3 Five-Year Survival Rates (%) According to Tumor Size and Axillary Lymph Node Status
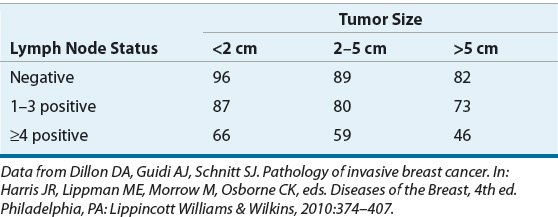
The number of affected lymph nodes is directly related to the risk of disease recurrence. The revised staging system for breast cancer recognizes the absolute number of positive nodes as a prognostic factor: N1 represents one to three positive nodes, N2 represents four to nine positive nodes, and N3 represents 10 or more positive nodes in its pathologic staging system.41 The relationship between tumor size and lymph node status is complex and not a simple grouping (see discussion below).
Certain histologic subtypes and clinical presentation of breast cancer have prognostic importance. As mentioned earlier, because women with pure tubular or mucinous tumors have more favorable outcomes than those with invasive ductal carcinomas, treatment recommendations may differ.48 Inflammatory breast cancer, although a clinical designation and not a distinct histologic subtype, is associated with a poor prognosis.44
Nuclear grade and tumor (histologic) differentiation are known independent prognostic indicators. Several histologic grading systems have been developed, most of which grade tumors with a score from 1 to 3: grade 1, well differentiated; grade 2, moderately differentiated; and grade 3, poorly differentiated. Higher grade tumors are associated with higher rates of distant metastasis and poorer survival. This factor aids in making treatment decisions, particularly for patients with small tumors and negative lymph nodes.
Lymphatic and vascular invasion (LVI), defined as evidence of tumor emboli in lymphatic or vascular spaces, is a poor prognostic factor likely representing ability of the cancer to spread via hematogenous routes. However, the utility of this as a prognostic factor is largely unknown and is not currently included in either staging or treatment guidelines.41,48
The rate of tumor cell proliferation also is associated with risk of breast cancer recurrence. Rate of cell proliferation can be evaluated with various techniques, including (1) mitotic index, which counts the number of mitotic bodies; (2) thymidine-labeling index or S-phase fraction with DNA flow cytometry, which determines the percentage of tumor cells actively dividing; or (3) the use of monoclonal antibodies (MoABs) to antigens present on proliferating cells, such as Ki-67. In a meta-analysis of 85 studies and nearly 33,000 patients, proliferation markers (including Ki-67, mitotic index, proliferating cell nuclear antigen, and thymidine or bromodeoxyuridine labeling index) were associated with significantly shorter disease-free and OS periods.58 These proliferation indices are additional factors that may be useful in decision making and may predict for responsiveness to chemotherapy, although this is still controversial.
Hormone receptors are not strong prognostic markers but are used clinically to predict response to endocrine therapy. Hormone receptors are nuclear transcription factors that, upon ligand binding, activate a variety of signal transduction pathways that result in cell growth and proliferation. Determination of both ER and progesterone receptor (PR) status is an established procedure that is important in the management of breast cancer. Immunohistochemistry is used to determine the level (i.e., quantity) of hormone receptors, which is important for predictive ability. Other methods of determining ER and PR status, such as mRNA expression, are under investigation but have not been validated as predictive markers. Hormone receptors are most valuable in predicting response to endocrine therapy. About 60% to 70% of patients with ER-positive and PR-positive tumors will respond to hormonal manipulation. More recently, the importance of PR has come under question because response to tamoxifen has been shown to be related to ER status independent of PR status.4 Guidelines for testing of ER and PR status are available and recommend standards for what tumors to test and methodologic guidelines for pathologists.59 About 50% to 70% of patients with primary or metastatic breast cancer have hormone receptor–positive tumors. Hormone receptor positivity, more common in postmenopausal women, is associated with a higher response to endocrine therapy and a longer DFS.
The HER2/neu (HER2) gene is located on chromosome 17q21 and encodes a 185-kilodaton transmembrane tyrosine kinase growth factor receptor. The HER2 protein is normally expressed at low levels in the epithelial cells of normal breast tissue. HER2 is a member of the HER growth factor receptor family, and its overexpression is associated with transmission of growth signals that control aspects of normal cell growth and division. HER2 overexpression occurs in about 20% to 30% of breast cancers and is associated with increased tumor aggressiveness, increased rates of recurrence, and increased mortality rates. In some studies, HER2 gene amplification and protein overexpression, measured by fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC), respectively, correlates with factors associated with a poor prognosis. HER2-positive status clearly predicts response to trastuzumab, which is a MoAB directed against the extracellular domain of the HER2 receptor. Tumors that are either IHC 3+ or FISH positive for gene amplification are considered to be positive for HER2.60 For equivocal results of IHC (2+) or FISH, confirmatory testing with the alternate test is recommended. HER2 gene amplification or protein overexpression has traditionally been considered a poor prognostic factor. However, more recent data suggest that patients with HER2-positive metastatic breast cancer treated with trastuzumab have improved survival rates compared with patients with HER2-negative metastatic breast cancer or patients with HER2-positive metastatic breast cancer who do not receive trastuzumab.61 These results demonstrate the powerful impact trastuzumab therapy has made on improving patient outcomes.
Although there is a growing understanding of the prognostic significance of individual factors, it is not clear how each factor contributes to the overall prognosis for an individual patient. Computer-aided models, including Adjuvant! (www.adjuvantonline.com), are available that combine patient- and tumor-related variables to estimate overall prognosis for individual patients with early stage breast cancer and aid in decisions regarding adjuvant systemic therapy.62 Such programs have limitations and should be used by healthcare professionals and not directly by patients because of the importance of accurate data entry, selection of different treatment options, and appropriate interpretation of results (see Systemic Adjuvant Therapy later).
Genetic profiling is also being used to provide prognostic and predictive information on clinical outcomes of breast cancer.62 The Oncotype DX assay uses a reverse-transcription polymerase chain reaction (RT-PCR) assay of 21 genes to predict the likelihood of distant recurrence in lymph node–negative and ER-positive breast cancer patients treated with tamoxifen. MammaPrint is another molecular prognostic test that uses DNA microarray analysis to measure the activity of a set of 70 genes to determine the likelihood of breast cancer recurrence in women with stage I or II breast cancer, tumor size 5 cm or smaller, and no lymph node involvement. Further details on these assays are available in the Systemic Adjuvant Therapy section later.
Novel molecular markers that have shown prognostic and predictive significance include urokinase-type plasminogen activator and its inhibitor, plasminogen activator inhibitor type 1, cyclin E, and the presence of tumor cells in bone marrow or circulating blood.63 Prospective validation studies will determine whether these tests can be used to assist decision making in individual patients.
In summary, lymph node status and tumor size are two significant prognostic factors that assist clinicians in estimating prognosis and making treatment recommendations for most breast cancer patients (see also Systemic Adjuvant Therapy later). Although the risk of recurrence is clearly high in patients with large primary tumors or lymph node–positive disease, many patients with small primary tumors and lymph node–negative disease will still develop metastases, yet our ability to accurately identify these individual patients is limited. Evaluation of additional prognostic factors can help identify which patients will have a good outcome with local therapy alone and which patients with aggressive features who would benefit from more aggressive, multimodality treatment. Despite these markers, a large proportion of patients will likely be treated unnecessarily with systemic adjuvant therapy (see later discussion), and better prognostic and predictive tools are needed to better select patients to undergo these toxic and costly treatments and procedures.
TREATMENT
Early Breast Cancer (Stage I and II)
Desired Outcomes
The desired therapeutic outcome of adjuvant therapy of breast cancer differs significantly from that of metastatic disease. Adjuvant therapy—chemotherapy, biologic therapy, and hormonal therapy—is administered with curative intent. The rationale for adjuvant therapy is that breast cancer, even when diagnosed in early stages when clinical evidence of distant spread is not apparent, is a systemic disease that spreads early to distant sites. Adjuvant therapy is intended to eradicate micrometastases and thus cure the patient of breast cancer. A predetermined number of cycles of adjuvant therapy or years of biologic or hormonal therapy (or both) are administered. The goals of neoadjuvant therapy are to eradicate micrometastatic disease, determine prognosis, and potentially conserve the breast tissue for a better cosmetic result. Adjuvant and neoadjuvant chemotherapy is often associated with significant toxicity. Clinicians and patients must weigh the short- and long-term risks of chemotherapy, biological therapy, and endocrine therapy with the benefits of lowering the risk of breast cancer recurrence.
Locoregional Therapy
![]() Most patients presenting with breast cancer today have an in situ tumor, a small invasive tumor with negative lymph nodes (stage I), or a small invasive tumor with axillary lymph node involvement (stage II). Surgery alone can cure most, if not all, patients with in situ cancers; 70% to 80% of patients with stage I; and about half of all patients with stage II cancers. The choice of surgical procedures has changed drastically over the past 50 years. This is partly a result of changes in our understanding of the biology of breast cancer and is partly a result of a series of well-conducted clinical trials performed over this time period.
Most patients presenting with breast cancer today have an in situ tumor, a small invasive tumor with negative lymph nodes (stage I), or a small invasive tumor with axillary lymph node involvement (stage II). Surgery alone can cure most, if not all, patients with in situ cancers; 70% to 80% of patients with stage I; and about half of all patients with stage II cancers. The choice of surgical procedures has changed drastically over the past 50 years. This is partly a result of changes in our understanding of the biology of breast cancer and is partly a result of a series of well-conducted clinical trials performed over this time period.
Over the years, many trials have investigated reducing the amount of surgery required to maintain acceptable cosmetic results and rates of local and distant recurrence and mortality. Breast-conserving therapy (BCT) includes removal of part of the breast, surgical evaluation of the axillary lymph node basin, and radiation therapy to the breast. The amount of breast tissue removed as a part of BCT varies from just removing the cancerous “lump” (a lumpectomy) with a small margin of adjacent normal-appearing tissue to removing the “lump” with a wider excision of adjacent normal-appearing tissue (a wide local excision) to removing the entire quadrant of the breast that includes the cancerous “lump” (a quadrantectomy). All of these techniques are referred to as a segmental or partial mastectomy. A meta-analysis of 18 clinical trials in almost 10,000 women found no difference in OS for patients who received BCT compared with mastectomy.64 However, this and other meta-analyses have suggested the potential for a small increase in the risk of locoregional recurrence with BCT.64,65
Most patients diagnosed today with breast cancer can be treated with BCT. Several factors should be considered in selecting patients for BCT, including any additional risk the remaining breast tissue poses despite the local effects of radiation therapy. The National Comprehensive Cancer Network (NCCN) recommends that women who carry a known BRCA1 or BRCA2 mutation undergo mastectomy and consider additional risk reduction strategies (e.g., bilateral mastectomies).48 Bilateral total mastectomy and oophorectomy reduce the risk of breast cancer occurrence in patients with BRCA1 or BRCA2 mutations, but both breast and ovarian cancers have been reported in patients who have had prophylactic removal of these organs. Multiple sites of cancer within the breast and the inability to attain negative pathologic margins on the excised breast specimen are predictive for an increased risk of recurrence with BCT and are indications for mastectomy. Some preexisting collagen vascular diseases (e.g., scleroderma, systemic lupus erythematosus) are relative contraindications for the use of BCT because of an increased risk of radiation-related adverse effects. Although local recurrence after BCT has not been consistently associated with an increased mortality rate, it is distressing to the patient and requires surgical removal of the breast. In addition, reconstructive therapy is often not feasible in a breast that has previously received irradiation. Another major consideration in selecting patients for BCT is the expected cosmetic result. For some patients, preservation of a limited amount of breast tissue may not justify the inconvenience of radiation therapy. Another approach to therapy for these patients is primary (neoadjuvant) systemic therapy to potentially shrink the tumor and minimize surgery (see the Systemic Adjuvant Therapy and Locally Advanced Breast Cancer sections for further details). Aside from the probability of local recurrence and the ability to achieve a satisfactory cosmetic result, consideration must be given to the availability of an external-beam radiation facility and the patient’s willingness to comply with the prescribed course of radiotherapy. In most instances, external-beam radiation therapy used in conjunction with BCT involves 4 to 6 weeks of radiation therapy directed to the entire breast tissue (typically a total of 50 Gy [5000 Rad] administered in 25 daily doses Mondays through Fridays with an optional boost of radiation to the tumor bed) to eradicate residual disease. Complications associated with radiation therapy to the breast are generally minor and include reddening and erythema of the breast tissue and subsequent shrinkage of the total breast mass beyond that predicted on the basis of breast tissue removal. Clinical trials are investigating the use of accelerated partial breast irradiation, intraoperative radiotherapy, or no radiation after segmental mastectomy for certain patient populations with a very low risk of recurrence.66 Until the results of these studies are available, the standard approach to BCT includes full-breast radiation therapy.
Postmastectomy radiation therapy to the chest wall may also be required in certain situations when tumors are large or the number of positive axillary lymph nodes is high (see the Locally Advanced Breast Cancer section). However, these criteria are also widely debated and are the subject of several meta-analyses. Despite the controversy, it is clear that some women may benefit from local radiation therapy even after removal of the entire breast (i.e., total mastectomy). The NCCN Guidelines state that women with four or more positive axillary lymph nodes should undergo postmastectomy radiation therapy. Patients with one to three positive ipsilateral axillary lymph nodes should strongly consider postmastectomy radiation, although conflicting data exist in this patient population.48 Patients with (a) positive surgical margins, (b) a tumor larger than 5 cm, or (c) tumors less than 5 cm with close margins (<1 mm of normal adjacent tissue) should consider postmastectomy chest wall radiation therapy. Finally, patients with surgical margins of at least 1 mm, tumor size of 5 cm or less, and negative axillary lymph nodes do not require postmastectomy chest wall radiation therapy. The optimal sequence of radiation therapy and chemotherapy is somewhat controversial. Concurrent administration of chemotherapy and radiation therapy is usually avoided because of an increase in local adverse effects. Most clinicians administer systemic chemotherapy immediately after surgery (if chemotherapy was not administered before surgery) given the hypothetical presence of systemic micrometastases that cannot be eradicated by local radiation therapy. Radiation therapy is then administered after chemotherapy, leaving hormone therapy (which is given for many years) for the end (see the Adjuvant Biologic Therapy section for a discussion of sequencing trastuzumab).
Accurate assessment of the spread of breast cancer cells to the axillary lymph nodes is critical for prognosis and the determination of the utility of both local and systemic treatments. ALND with histopathologic study of the full axillary specimen, including level I and II lymph nodes, was the gold standard for detecting axillary nodal involvement and determining the number of lymph nodes containing tumor. The number of positive axillary lymph nodes remains the most powerful predictor of breast cancer recurrence and survival, but other benefits may include a therapeutic effect of removing the lymph nodes and obtaining information to guide treatment selection. However, axillary dissection is associated with significant morbidity, with an acute complication rate as high as 20% to 30% and rates of chronic lymphedema as high as 20% to 30%.67,68 Recent studies indicate that about 60% of patients with early stage breast cancer present with lymph node–negative disease, which indicates that many women would derive no therapeutic benefit but would be exposed to the complications from the procedure.
For these reasons, a procedure involving lymphatic mapping and SLNB is recommended at many centers across the United States, and guidelines regarding recommendations for this procedure are now available.69 The sentinel lymph node(s) is the first lymph node(s) that receives lymph drainage from the primary tumor. Injection of a vital blue dye, a radiocolloid, or both around the primary breast tumor identifies the sentinel lymph node(s) in most patients, and the status of this lymph node(s) may predict the status of the remaining nodes in the nodal basin. Patients with lymph nodes that are suspicious for cancer involvement either by physical examination or imaging should have a biopsy performed to exclude lymph node involvement. SLNB has become the standard of care for patients with clinically negative axillary lymph nodes.48 Patients with a positive sentinel node or in whom the sentinel node is not identified should proceed to a level I and II ALND, although ALND after a positive lymph node found with SLNB is also controversial in certain patient populations. Data from a single randomized trial suggest that ALND after SLNB in women with clinically node negative tumors smaller than 5 cm, fewer than three involved sentinel lymph nodes, and undergoing BCT with subsequent breast irradiation resulted in higher morbidity, no improvement in local recurrence, and no difference in DFS or OS with SLNB alone.70
Despite differences in the mapping technique, the experience of the surgeon, or the patient populations studied, recent studies show that this approach identified the sentinel lymph node(s) in more than 90% of patients.71 In studies that incorporated completion axillary dissections for comparison, the SLNB procedure accurately predicted the status of the remaining axillary nodes in more than 90% of patients. Considerable controversy exists over the use of this procedure in women with large tumors (>5 cm) or locally advanced disease, palpable axillary lymph nodes, a multifocal or multicentric breast tumor, prior neoadjuvant (preoperative) chemotherapy, or prior surgery involving the breast or axilla. Patients who are pregnant or lactating are generally not considered candidates for this procedure because of concerns regarding the effects of the blue dye or the radiocolloid on the fetus. The decision of whether to use the sentinel lymph node procedure or a full axillary dissection is complex, and readers are referred to an excellent review for further information.72
Simple or total mastectomy involves removal of the entire breast without dissection of the underlying muscle or axillary nodes. The major disadvantage of this procedure is that axillary nodal status is not determined, and therefore important prognostic information may be lost. This procedure is used in patients with carcinoma in situ, in whom there is a 1% incidence of axillary node involvement, or in cases of in-breast recurrences after BCT.
The early trials investigating less extensive surgical approaches to breast cancer are widely credited with the finding that BCT is an appropriate primary therapy for most women with stages I and II disease and is preferable because it arguably provides survival rates equivalent to those of modified radical mastectomy. These historical trials provided valuable information regarding the natural history of the disease and identified pathologic prognostic factors associated with early cancer spread. The preponderance of information available regarding selection of women most likely to benefit from systemic adjuvant therapy was derived from pathologic evaluation of tissues archived from these early trials. It is hoped that further investigation into less extensive local therapy (now focused on the surgical approach to the axilla and radiation therapy) will continue to provide valuable information for the future.
Systemic Adjuvant Therapy
![]() Systemic adjuvant therapy is defined as the administration of systemic therapy after definitive local therapy (surgery, radiation, or a combination of these) when there is no evidence of metastatic disease but a high likelihood of disease recurrence. By the time breast cancers become clinically detectable, they have likely been present for a number of years and have had ample opportunity to establish distant micrometastases. Micrometastatic disease can travel from the primary breast tumor and spread to distant organs through several different routes (e.g., hematogenous spread through blood vessels, lymphangitic spread through lymph channels, local extension to surrounding structures). Because local therapies such as breast surgery and irradiation do not address distant micrometastases, systemic therapy may be required to target these tumor cells that have escaped the local area of the breast. The likelihood of micrometastatic disease presence is used to attempt to identify patients with a high risk of recurrence who would require systemic adjuvant therapy. Many collaborative research groups have conducted step-wise series of studies designed to identify appropriate candidates for systemic adjuvant therapy and the optimal regimens and duration of therapy. Several hundred randomized clinical trials evaluating various systemic adjuvant modalities have been reported. Most published results confirm that administration of chemotherapy, endocrine therapy, or both, results in improved DFS or OS for all treated patients or more commonly for patients in specific prognostic subgroups (e.g., nodal involvement, menopausal status, hormone receptor status, or HER2 status). The huge amounts of data generated by these trials have resulted in a great deal of controversy, with different conclusions being reached by various experts.
Systemic adjuvant therapy is defined as the administration of systemic therapy after definitive local therapy (surgery, radiation, or a combination of these) when there is no evidence of metastatic disease but a high likelihood of disease recurrence. By the time breast cancers become clinically detectable, they have likely been present for a number of years and have had ample opportunity to establish distant micrometastases. Micrometastatic disease can travel from the primary breast tumor and spread to distant organs through several different routes (e.g., hematogenous spread through blood vessels, lymphangitic spread through lymph channels, local extension to surrounding structures). Because local therapies such as breast surgery and irradiation do not address distant micrometastases, systemic therapy may be required to target these tumor cells that have escaped the local area of the breast. The likelihood of micrometastatic disease presence is used to attempt to identify patients with a high risk of recurrence who would require systemic adjuvant therapy. Many collaborative research groups have conducted step-wise series of studies designed to identify appropriate candidates for systemic adjuvant therapy and the optimal regimens and duration of therapy. Several hundred randomized clinical trials evaluating various systemic adjuvant modalities have been reported. Most published results confirm that administration of chemotherapy, endocrine therapy, or both, results in improved DFS or OS for all treated patients or more commonly for patients in specific prognostic subgroups (e.g., nodal involvement, menopausal status, hormone receptor status, or HER2 status). The huge amounts of data generated by these trials have resulted in a great deal of controversy, with different conclusions being reached by various experts.
![]() Interpretation of results of systemic adjuvant therapy is difficult because of differences in the patient populations studied, the variation in natural history of breast cancer, the absence of information regarding pathologic prognostic factors in many studies, and differences in treatment approach and methods of analysis. Several groups around the world have conducted meta-analyses of similar breast cancer trials in hopes of gaining more insight regarding adjuvant systemic therapy than a single study can provide. One such effort, organized by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), is based on a worldwide collaboration involving multiple randomized trials and is continually updated with results from new clinical trials. The EBCTCG’s overview analyses are updated periodically as new data become available. The most recent updates, published in 2011 and 2012, reflect the long-term effects on breast cancer recurrence and survival for adjuvant tamoxifen and chemotherapy, respectively.4,5 Many important questions regarding the optimal way to administer adjuvant chemotherapy and endocrine therapy and the magnitude of benefit as measured by DFS or OS in clinically relevant subsets of patients have been answered by these overview analyses. Simply stated, the results of these analyses support the use of adjuvant endocrine therapy in all patients with positive hormone receptor status regardless of age, menopausal status, involvement of axillary lymph nodes, or tumor size.4 The results of these overview analyses also support the use of adjuvant chemotherapy in most women with lymph node metastases or with primary breast cancers larger than 1 cm in diameter (both node negative and node positive).5 It is important to note that data from clinical trials incorporating modern AIs or trastuzumab into adjuvant regimens are not included in these analyses because sufficient long-term follow-up has not been reached. Results from these more recent clinical trials are discussed later (see the Adjuvant Biologic Therapy and Adjuvant Endocrine Therapy sections).
Interpretation of results of systemic adjuvant therapy is difficult because of differences in the patient populations studied, the variation in natural history of breast cancer, the absence of information regarding pathologic prognostic factors in many studies, and differences in treatment approach and methods of analysis. Several groups around the world have conducted meta-analyses of similar breast cancer trials in hopes of gaining more insight regarding adjuvant systemic therapy than a single study can provide. One such effort, organized by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), is based on a worldwide collaboration involving multiple randomized trials and is continually updated with results from new clinical trials. The EBCTCG’s overview analyses are updated periodically as new data become available. The most recent updates, published in 2011 and 2012, reflect the long-term effects on breast cancer recurrence and survival for adjuvant tamoxifen and chemotherapy, respectively.4,5 Many important questions regarding the optimal way to administer adjuvant chemotherapy and endocrine therapy and the magnitude of benefit as measured by DFS or OS in clinically relevant subsets of patients have been answered by these overview analyses. Simply stated, the results of these analyses support the use of adjuvant endocrine therapy in all patients with positive hormone receptor status regardless of age, menopausal status, involvement of axillary lymph nodes, or tumor size.4 The results of these overview analyses also support the use of adjuvant chemotherapy in most women with lymph node metastases or with primary breast cancers larger than 1 cm in diameter (both node negative and node positive).5 It is important to note that data from clinical trials incorporating modern AIs or trastuzumab into adjuvant regimens are not included in these analyses because sufficient long-term follow-up has not been reached. Results from these more recent clinical trials are discussed later (see the Adjuvant Biologic Therapy and Adjuvant Endocrine Therapy sections).
Clinicians need to understand the relative and absolute magnitude of benefit associated with adjuvant systemic therapy in breast cancer if they are to help patients understand their treatment options and make informed decisions regarding their care. A proportional reduction of 25% might equivalently be described as an odds ratio (OR), RR ratio, or HR of 0.75; a RR or odds reduction of 25%; or a 25% reduction in the risk of death or death rate. For a given proportional reduction in death rate, the absolute improvement in 15-year survival will depend on the baseline risk of death with no treatment, which varies based on prognostic factors that include patient characteristics, disease characteristics, and biomarkers identified earlier in this chapter. Table 105-4 shows the number of deaths avoided per 100 patients treated in several hypothetical subsets of patients with different estimated 15-year survivals without adjuvant therapy as a function of different estimates of treatment benefit shown as the proportional reductions in mortality if they did receive adjuvant therapy. About 15 of every 100 patients benefited at 15 years from adjuvant therapy when a 30% proportional reduction in mortality is observed in the highest risk subgroups (50% death rate with no adjuvant therapy). In contrast, the same 30% proportional reduction in mortality translated into a benefit for only three of 100 patients in the lowest risk subset (10% death rate with no adjuvant therapy). Thus, the absolute benefit of adjuvant therapy depends on both the proportional reduction in mortality and the risk of disease recurrence, with the greatest benefit observed in the highest risk treatment groups.
TABLE 105-4 Absolute Reduction in Mortality at 10 Years per 100 Patients Treated