Breast
Louis P. Dehner, M.D.
The breast shares a similar sequence of developmental-morphologic events with the skin, salivary gland, tooth, kidney, and lung. There is an initial proliferation of surface epithelium that subsequently projects into the underlying mesenchyme. Molecular interaction between the epithelium and mesenchyme results in the branching of the epithelium that in the female breast gives rise to a network of ducts and terminal duct lobular units, which only develop with puberty. The first vestige of the breast is seen in the 4th week of gestation when small mounds of surface ectoderm form as placodes; these paired structures are found along a line from the anterior thorax into the inguinal region. The eventual number of mammary glands as functional structures varies among the mammalian species and correlates with number of offspring in the litter (1). During the 5th week of gestation, the placode in a cap of pseudostratified epithelium proliferates into the stroma where the epithelium and mesenchyme interact through an intricate series of subcellular programs (2). The mammary buds remain as solid structures until approximately the 10th week when branching is initiated and secondary buds are formed; this process of lengthening and branching continues throughout gestation (3). There is also narrowing of the neck of the bud with the transformation of the solid epithelial structures into structures with a central lumen during the 20th week of gestation; the latter structures are the lactiferous ducts, which are recognized by 32 weeks when they open to the surface of the skin. By 28 weeks, the two primary cell types of the ducts and acini have differentiated into a basal myoepithelial cell and luminal cell.
The mesenchyme is the other central component not just from the perspective of forming supporting structures like blood vessels, but it has an early inductive effect upon the overlying ectoderm during the phase of placode and bud formation via paracrine and endocrine receptors (4). The sprouting of the bud by parathyroid hormone-related peptide signaling and its receptor is a function of mesenchyme. The adipose tissue of the breast is a necessary component for the mediation of future growth also through signaling cascades (5). A complex molecular network is in place in both the stroma and epithelium to interpret and coordinate cytokines and growth factors including hormones and hormone receptors (6).
Several signaling pathways are involved in the earliest stages of placode and bud formation (2). These same pathways are activated in the regulation of development in virtually all organ systems; these pathways include hedgehog, Wnt/β-catenin, TGF-β, and FGG pathways (7,8,9).
A term male or female newborn often has subareolar nodules measuring 2 to 3 cm in greatest dimension (Figure 20-1). Galactorrhea may be noted as well. As placental-maternal hormones and prolactin from the infant decline in the first month, the epithelial proliferation ceases and the stroma regresses with the resolution of the nodules. Until the onset of puberty, the breast tissue remains in a resting or quiescent state in the absence of a functioning neoplasm of the adrenal cortex, ovary (juvenile granulosa cell tumor), testis (Leydig cell tumor), or pituitary (prolactinoma).
With puberty, the female breast with its rudimentary ductal network develops an extensive network of ducts through the mechanism of branching morphogenesis (10). There is penetration of the stroma where the process is continuously monitored and constrained through the various signaling pathways. Unlike the earlier stages of breast development, estrogen and insulin-like growth factor 1 (IGF1) are critical in the growth of breasts at puberty and with the formation of the terminal duct lobular unit (5,7,11). The inhibition of IGF1 by its binding protein results in apoptosis of ductal epithelial cells, whereas IGF1 facilitates the growth in the number of terminal end buds (7,12). Other molecular agents important in breast organogenesis and growth include growth hormone, TGF-α, TGF-β, tenascin-c, and collagen type IV (13).
Morphologic events at thelarche with progressive growth of the breast through its five Tanner stages involve the network of ducts as they branch and form acinar-lobular units from the terminal ducts (11,14). Fibrous stroma and adipose tissue constitute 25% or more of the total breast volume. There is progressive proliferation and condensation of fibrous stroma around the ducts, a process initially recognized in the embryo-fetal stage of breast development (11,15).
CONGENITAL ANOMALIES
Abnormalities in breast development may or may not be accompanied by defects in the chest wall. There are several categories of breast anomalies based upon complete absence or reduction in the breast volume (amastia or hypoplasia to varying degrees) that may be unilateral or bilateral, defects in the nipple and/or areolar complex (polythelia or athelia), accessory breast tissue in the axillary tail or vulva, and abnormal shape and anomalies in the chest wall. If one reviews the development of the normal breast, it is possible to associate the particular abnormality with a perturbation in one of the sequence of events in normal mammary gland embryology. Several reviews were found helpful for those with a particular interest in this topic (15,16,17,18,19).
Congenital Absence of the Breast
Absence of the breast is defined as total absence of breast parenchyma or amastia, which includes both the nippleareolar complex and underlying mammary tissue (amastia), and absence of the nipple (athelia) and mammary gland tissue (amazia) (16). Although most cases are sporadic and may be unilateral or bilateral, there are several syndromic associations, of which the Poland syndrome may be the most familiar (16,20,21). These children have aplasia/hypoplasia of the pectoralis major and minor muscles, and the nipple and breast tissue are absent (22). Yet another syndrome is characterized by congenital choanal atresia and bilateral amastia (23). Scalp-ear-nipple syndrome (Finlay-Marks syndrome), an ectodermal dysplasia-like disorder, has a missense mutation in the KCTDI gene (24). Athelia and/or amastia is present in these children, which would be anticipated in a gene that is involved in ectodermal development. Meier-Gorlin syndrome with mutations in ORC1, ORC4, ORC6, CDT1, and CDC6 genes is accompanied by amazia in 100% of cases (25,26). The topic of bilateral congenital amazia has been thoroughly reviewed elsewhere (27).
Athelia with a congenital absence of the nipple-areolar complex is invariably syndromic and usually a manifestation of one of the ectodermal dysplasias; there is also absence of glandular mammary tissue (25,28). Limb-mammary syndrome in addition to congenital amastia, but unlike the p63 syndrome, does not have mullerian tract abnormalities (29). A lack of breast development has been reported in the genetic disorder, 17-alpha-hydroxylase/17, 20-lyase deficiency (30). The absence of breast development should initiate studies, if not already started, on one of several possible genetic disorders. In the case of an ectodermal dysplasia, there is not only absence of mammary breast tissue but also cutaneous adnexal structures that are absent or severely miniaturized (31,32,33).
Breast Displacement
Breast displacement on the anterior chest wall resulting in asymmetry is yet another anomaly. It is probably not true that the distance between the nipples is increased in Turner or monosomy 45X0 syndrome. By contrast, congenital symmastia is a disorder in which the breasts are displaced medially by the presence of fibrous bands (34).
Polythelia and Supernumerary Breast Tissue
Polythelia, also known as supernumerary nipples, is relatively common with a frequency of 0.2% to 5.6% and may be unilateral or bilateral (10% of cases) and is found along the embryonic milk line to the level of the vulva (35). Newborn males are found with polythelia somewhat more commonly than are females. Urinary tract abnormalities have been reported in association with polythelia, but there is still some question as to the strength of this relationship in some quarters (36). Yet another association of polythelia is with various genodermatoses (37). These are also reported kindreds with polythelia (38). Microscopically, the supernumerary nipple has a somewhat papillomatous epidermis and has a resemblance to an epidermal nevus, in addition to hyperpigmentation of the basal layer, lactiferous ductlike structures with or without epithelial hyperplasia, pilosebaceous units with or without keratin accumulation, and cyst formation resembling a keratinous cyst of infundibular type.
Supernumerary or accessory breast tissue is found in 1% to 2% of the population where the two most common sites are the axilla and vulva, and bilateral accessory breast tissue is found in approximately 40% to 45% of these cases (39). Accessory breast tissue may remain occult until pregnancy with the development of an axillary or vulvar mass. The ductal lobular tissue is indistinguishable from orthotopic breast tissue. Fibroadenomas (FA) may arise in accessory breast in the adolescent female or a hidradenoma papilliferum in the vulva of a comparably aged female.
ACQUIRED BREAST PATHOLOGY
This section is concerned with a variety of pathologic processes, both common and uncommon and usually presenting as a discrete mass or as diffuse swelling in the breast. Most of these lesions make their clinical appearance in pubertal females as a FA or gynecomastia in the adolescent male. Together, these two entities account for 50% or more of all breast pathology in patients 20 years old or less (40). Beyond these two entities, a number of other lesions present in the breast of children and adolescents, but usually as single examples. An important point to keep in mind is that only 2% to 3% of all breast lesions in children are malignant (41).
Benign Fibroepithelial Neoplasms
Fibroepithelial neoplasms are biphasic tumors of the breast that arise from terminal duct lobular units and the intra- and extralobular stroma. The various individual entities among the fibroepithelial neoplasms are recognized morphologically as the FA with and without distinctive histologic features mainly in the appearance of the stroma (cellular, myxoid, and atypical stromal cells), benign phyllodes tumor (PT), low-grade malignant or borderline PT, and high-grade malignant PT (42). Sarcomatous overgrowth of a malignant PT with residual foci of the latter is considered as a tumor type in this spectrum of neoplasms. Yang et al. (43) have discussed and illustrated the overlap of these various fibroepithelial tumors. Carcinomas of lobular or ductal type are known to occur rarely in or around FAs, but these cases are restricted almost exclusively to adults (44).
Fibroadenomas
FA is the most common neoplasm of the breast in the first two decades of life, and the overwhelming majority of cases are diagnosed in the interval from early puberty into late adolescence and beyond. There is a predilection for these tumors to occur in young African and African American females in whom the tumor commonly presents as a rapidly enlarging mass, which may be multifocal and even bilateral (45). Both FA and PT are seemingly more common in several tumor predisposition syndromes including Li-Fraumeni, Maffucci, and Beckwith-Wiedemann syndromes and Carney complex (40,46).
In most cases, there are relatively few difficulties in the histopathologic recognition of an FA in a young patient, but there is a thicket of largely semantic issues in the distinction, if any, among the juvenile, cellular, and giant FA from the FA, ordinary, and benign PT since the clinical and pathologic criteria are seemingly not burdened by a consensus or established criteria (47,48). Such qualifications as “increased stromal cellularity,” “massive,” and “juvenile” are both vague and subjective in their application to FAs in young individuals between the ages of 12 and 20 years (49).
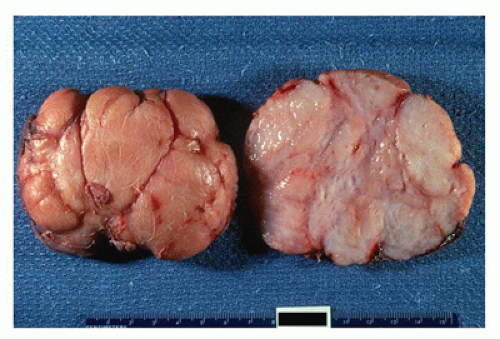
Most FAs are well circumscribed from the surrounding breast parenchyma with a seeming cleft between the tumor and adjacent soft tissues. A true capsule is not present in most cases. The external surfaces often have a bosselated appearance that corresponds to the bulging multinodular or lobulated cut surface (Figure 20-2). A glistening, mucoid yellowish-tan to whitish surface characterizes most tumors although extensive hemorrhage may indicate the presence of infarction (50). The majority of tumors measure 2 to 5 cm in greatest dimension although some have applied the designation of “giant” to those tumors measuring 5 cm or greater (up to 20 cm). Some of the larger FAs can ulcerate the overlying skin and present as a fungating mass (49). Two basic histologic patterns are recognized in FAs: an intracanalicular pattern with a prominent stroma compressing strands of epithelium in various configurations including the phyllodes or leaflike pattern of a benign PT (Figure 20-3) and the periductal or pericanalicular pattern whose stroma separates individual terminal ductal lobular units (Figure 20-4). One or the other of these patterns is exclusive in any one
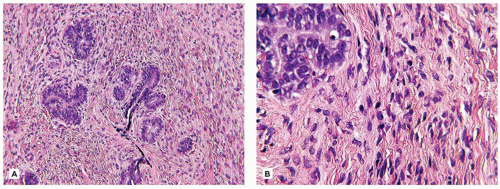
FA, or alternatively, there are FAs with a mixed pattern of phyllodes-like profiles and intracanalicular and/or pericanalicular patterns. The cellularity of the stroma is the usual source of concern in FAs in young individuals. In most cases, the stroma has a bland fibrous appearance with or without edema or a predominant myxoid stroma (Figure 20-5). The stroma adjacent to the epithelium is typically more cellular and less fibrotic than is the more remote, fibrous stroma. The occasional mitotic figure can be found in the stroma adjacent to the epithelium. When the stroma is more uniformly cellular, attention is directed to the presence or absence of mitotic figures since the latter separates the so-called cellular or juvenile FA from the borderline or low-grade PT (Figure 20-6A, B). Mitotic activity generally should not exceed 2 mitoses per 10 high-power (400×) fields (HPF) nor the leaflike phyllodes epithelial pattern, but in a needle core biopsy, the latter epithelial configuration may be difficult to appreciate (51). Mitotic figures become a concern when they are found in the stroma without difficulty, which usually translates into 10 mitoses in 10 HPF. An infiltrating stromal border at the periphery should be viewed with concern. Pleomorphic stromal giant cells are found infrequently, similar in some respects, to those stromal cells in nasal polyps or lamina propria of the bladder (52).
FA, or alternatively, there are FAs with a mixed pattern of phyllodes-like profiles and intracanalicular and/or pericanalicular patterns. The cellularity of the stroma is the usual source of concern in FAs in young individuals. In most cases, the stroma has a bland fibrous appearance with or without edema or a predominant myxoid stroma (Figure 20-5). The stroma adjacent to the epithelium is typically more cellular and less fibrotic than is the more remote, fibrous stroma. The occasional mitotic figure can be found in the stroma adjacent to the epithelium. When the stroma is more uniformly cellular, attention is directed to the presence or absence of mitotic figures since the latter separates the so-called cellular or juvenile FA from the borderline or low-grade PT (Figure 20-6A, B). Mitotic activity generally should not exceed 2 mitoses per 10 high-power (400×) fields (HPF) nor the leaflike phyllodes epithelial pattern, but in a needle core biopsy, the latter epithelial configuration may be difficult to appreciate (51). Mitotic figures become a concern when they are found in the stroma without difficulty, which usually translates into 10 mitoses in 10 HPF. An infiltrating stromal border at the periphery should be viewed with concern. Pleomorphic stromal giant cells are found infrequently, similar in some respects, to those stromal cells in nasal polyps or lamina propria of the bladder (52).
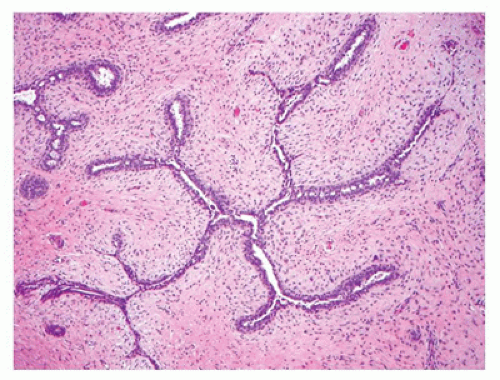 FIGURE 20-3 • Fibroadenoma with the intracanalicular pattern of stroma compressing the epithelial profiles. |
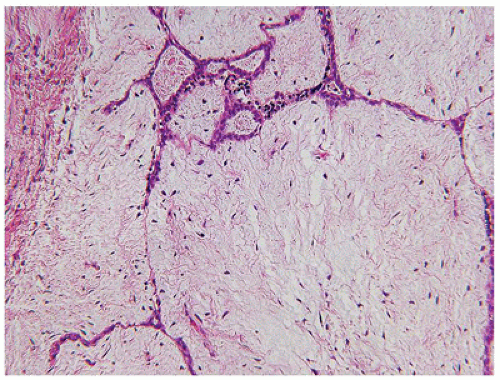
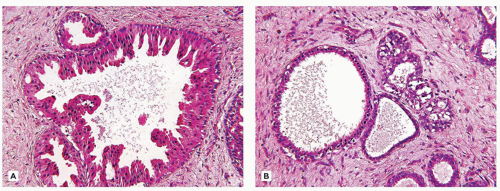
While there is considerable focus on the stroma of FAs, the epithelium may be quite hyperplastic with a degree of complexity and papillary profiles, which are especially notable in those tumors from adolescent or young adult women. Fibrocystic changes with cysts, apocrine metaplasia, and sclerosing adenosis are seen in some FAs in young individuals though they are more commonly observed in FAs from older women (Figure 20-7A, B). As a final note, there are examples of intraductal papillary lesions with FA-like features in young females (Figure 20-8) (53,54). Some have questioned the relationship in younger individuals between the intraductal papilloma and FA.
Immunohistochemistry has a very limited role in the diagnosis of FA. Those cases with an unsettling degree of cellularity and mitotic activity may benefit with Ki-67 and p53 staining (55). The stroma cells in FAs are rich in CD34+ fibroblasts, factor XIIIa+ dendrocytes, and smooth muscle actin + myofibroblasts (56). It has been noted that CD10+ stromal cells are uncommon in cellular FAs (57).
A recurrence rate of 10% to 15% is reported after resection of an FA in young patients; however, the “recurrence” may represent a new lesion. There are rare examples, principally in adults, of malignant progression of a recurrent FA to a malignant PT (58).
Benign PT is basically defined as a fibroepithelial neoplasm whose architecture is defined by the leaflike epithelial-lined structures and a stroma with a variable degree of cellularity (Figure 20-9). The periductal stroma is usually more cellular than in the stroma of the FA. An isolated mitotic figure in the mesenchymal cells adjacent to the epithelial component is permissible, which is also the case in an FA.
Adenomas
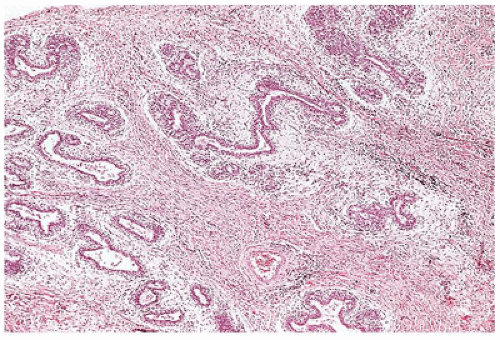
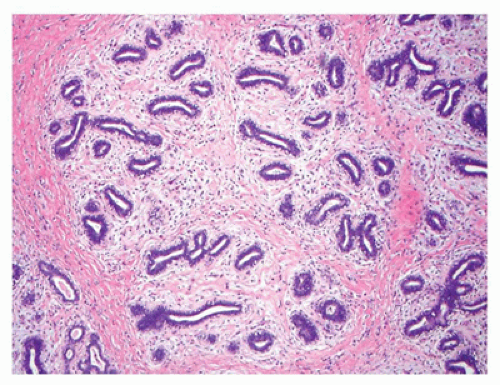
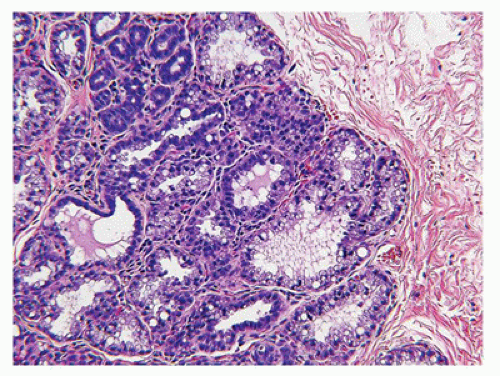
Adenomas constitute at least two general categories of breast lesions: one a monotypic fibroepithelial neoplasm arising from the terminal duct lobular unit in the absence of an intralobular stroma. The tubular and lactational adenomas are examples of the latter category (59,60). Tubular or pure adenoma is uncommon compared to FA, but is seen in the same young age group and is undistinguishable clinically from the FA (Figure 20-10). The distinctive histologic features are those of a multinodular tumor, which is composed of small uniform tubuloglandular structures with a variably prominent stroma in the background (Figure 20-11). In fact, some fibroepithelial neoplasms have a mixed pattern of tubular adenoma and FA. Lactational or secretory changes are the
features of the so-called lactational adenoma (Figure 20-12). Similar cellular changes are seen in the FA during pregnancy or in the postpartum period. Both the FA and tubular adenoma may uncommonly undergo partial or total infarction spontaneously in association with pregnancy, lactation, or following a fine needle biopsy (50,61).
features of the so-called lactational adenoma (Figure 20-12). Similar cellular changes are seen in the FA during pregnancy or in the postpartum period. Both the FA and tubular adenoma may uncommonly undergo partial or total infarction spontaneously in association with pregnancy, lactation, or following a fine needle biopsy (50,61).
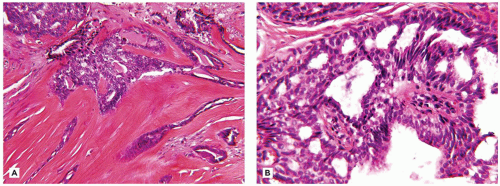
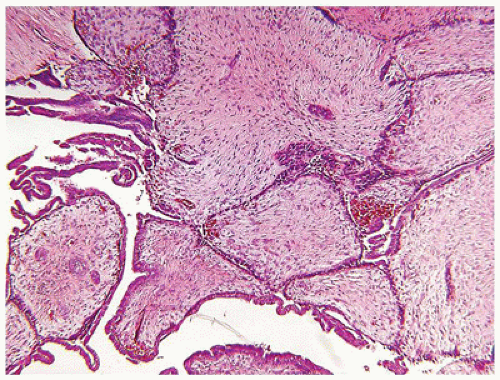 FIGURE 20-8 • Fibroadenoma-like features are seen in this intraductal papillary lesion in the right breast of an 11-yearold female. |
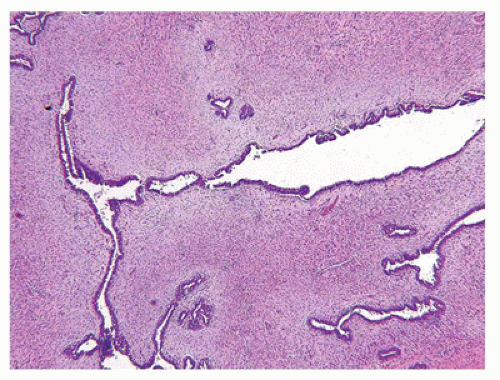 FIGURE 20-9 • Benign phyllodes tumor in the left breast of a 16-year-old female. The leaflike epithelial profiles are surrounded by a pale hypocellular and fibrous stroma. |
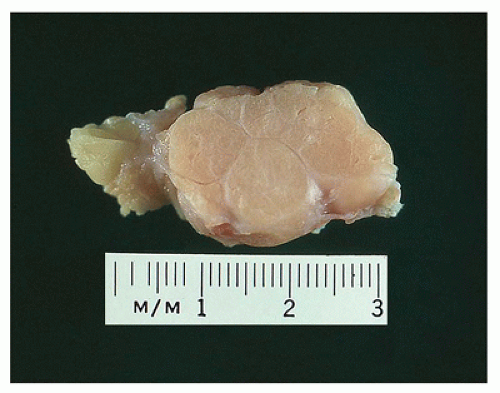 FIGURE 20-10 • Tubular adenoma has a glistening multilobulated cut surface. Note the sharp circumscription from the adjacent stroma. There is little to differentiate this gross appearance from an FA. |
The second category of adenomas are those tumors that are analogs, if not homologs, of sweat gland tumors of the skin whose occurrence is not all that surprising when one considers the common morphogenesis of the breast and skin (62). Syringomatous adenoma of the nipple, formerly nipple duct adenoma, is a rare rumor regardless of age at clinical presentation, but has been reported in later childhood (63). The microscopic appearance of elongated and tubular glands within a fibrous stroma with infiltrative features in the subareolar location can be mistaken for tubular-ductal carcinoma. At least in the setting of an older child or adolescent, the concern for malignancy should be quite low. Other sweat gland analog tumors of the breast constitute a spectrum from the typical syringocystadenoma papilliferum, eccrine spiradenoma, clear cell hidradenoma, and mixed tumor (pleomorphic adenoma).
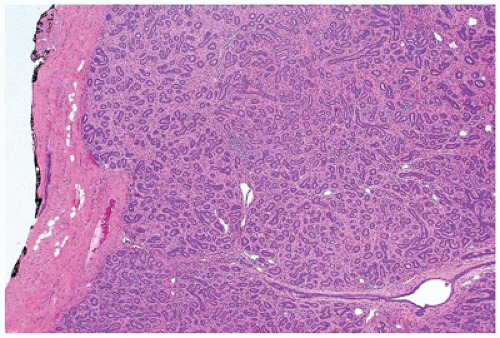 FIGURE 20-11 • Tubular adenoma has well-defined, lobulated borders. Some fibrous stroma is present around the uniform tubular gland and has pericanalicular-like features. |
Cysts and Ductal Papillary Lesions
Cysts and intraductal proliferative lesions whose clinical presentation with nipple discharge or as diffuse swelling of the breast in childhood are judged to be infrequent based upon reports in the literature and our own experience. Bloody nipple discharge in children can have several etiologies as reviewed by Imamoglu et al. (64). Another example of an intraductal proliferation is the intraductal FA, which was reported with infarction and hemorrhage in a 12-year-old female (65).
Galactocele, usually seen in women who are lactating, is documented in male children during the first 2 to 3 years of life (66,67). The dilated ducts are either empty or contain secretions rich in lipids with or without accompanying foamy macrophages. An apocrine epithelial lining is appreciated in the smaller ducts, whereas the epithelium is often compressed or inapparent in the larger ducts. Another similar process is mammary duct ectasia, which is seen in a somewhat broader age group than the galactocele and includes females and may be accompanied by periductal chronic inflammation (68).
Papillary epithelial proliferations encompass several lesions, all of which are uncommon in children, but one, juvenile papillomatosis or so-called Swiss cheese disease, is seen mainly in adolescent and young adult women. Another lesion, papillary ductal hyperplasia, which is differentiated from juvenile papillomatosis, also presents in adolescence. Two other papillary lesions are diagnosed predominantly in adults, intraductal papilloma and florid papillomatosis of the nipple, with its several patterns (69).
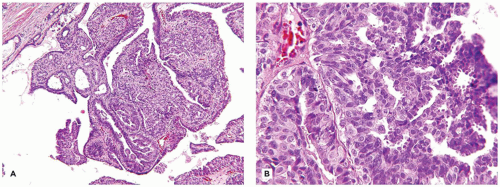
Intraductal papilloma may be a solitary lesion or involve several contiguous large ducts. Unlike its malignant counterpart, one of the hallmark features is the absence of a monomorphous pattern, but can harbor focal areas with more concerning features (Figure 20-13A, B). Intraductal papilloma arises in the lactiferous ducts as bulbous, papillary structures with more or less prominent stroma. The surface epithelium varies in the complexity of its pattern, and the underlying stroma contains tubulo-glandular profiles. The stroma is prominent and has a fibrosclerotic or edematous appearance (Figure 20-14A, B).
Juvenile papillomatosis presents as a mass in the breast and has been reported in infancy and in males (70). It has been seen in the setting of Noonan syndrome, and there is a higher-than-expected family history of carcinoma of the breast (69,71). Pathologically, the mass measuring 2 to 5 cm. in diameter is composed of macroscopically apparent cysts of varying sizes with a fibrous stroma and an intermixture of adipose tissue (Figure 20-15). The cysts have an exuberant papillary proliferation whose architecture resembles the complex intraductal papilloma and at the other extreme has a simple columnar or cuboidal epithelium with or without apocrine features (Figure 20-16A, B). Microcysts are interposed among the larger cysts, and calcifications are commonly present.
Papillary duct hyperplasia is a lesion in adolescent and young adult women, which often presents as a mass, often but not exclusively in the subareolar or central location in the breast. Multiple small to intermediate-sized ducts have a papillomatous proliferation with features of a papilloma, sclerosing papilloma, or papillomatosis with its more complex architecture (Figure 20-17) (72). Though some of these same features are found in juvenile papillomatosis, unlike the latter, there are no cysts, apocrine metaplasia, fibrosis, and chronic inflammation. However, we have seen cases where
overlapping microscopic features were present leading to some diagnostic semantic improvisation. Another pathologic process is subareolar sclerosing duct hyperplasia whose features overlap with the nipple duct adenoma or florid nipple duct papillomatosis.
overlapping microscopic features were present leading to some diagnostic semantic improvisation. Another pathologic process is subareolar sclerosing duct hyperplasia whose features overlap with the nipple duct adenoma or florid nipple duct papillomatosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree