Bone Biology in Health and Disease1
Robert P. Heaney
1Abbreviations: Al, aluminum; BMD, bone mineral density; [Ca2+], concentration of calcium ions; CT, calcitonin; DXA, dual-energy x-ray absorptiometry; ECF, extracellular fluid; OI, osteogenesis imperfecta; PTH, parathyroid hormone; RANKL, rank ligand.
BONE COMPOSITION AND STRUCTURE
Bone is a tissue in which cells make up only 2% to 5% of the volume, and nonliving material make up 95% to 98%. It is the nonliving material that gives the bone its basic mechanical properties of hardness, stiffness, and resiliency. This nonliving material consists of a mineral-encrusted protein matrix (also called osteoid), with the mineral comprising about half the volume and the organic matrix the other half. Unlike other connective tissues, virtually no free water is present in the bony material itself. Embedded in this solid material are cells, called osteocytes, residing in lacunae in the matrix and communicating with one another through an extensive network of long cellular processes lying in channels called canaliculi, which ramify throughout the bone. As a consequence of this arrangement, virtually no volume of normal bone is more than a few micrometers from a living cell. Furthermore, even in the dense bone of the shafts of long bones, an extensive network of vascular channels exists, so the most remote osteocyte is typically no more than 90 μm away from a capillary.
Bone Mineral
The mineral of bone is a carbonate-rich, imperfect hydroxy-apatite with variable stoichiometry. Calcium comprises 37% to 40%, phosphate 50% to 58%, and carbonate 2% to 8% of this mineral. These values vary somewhat from species to species, and the carbonate component is particularly sensitive to systemic acid-base status (decreasing in acidosis and increasing in alkalosis). In addition, bone mineral contains small amounts of sodium, potassium, magnesium, citrate, and other ions present in the extracellular fluid (ECF) at the time the mineral was deposited, adsorbed onto the crystal surfaces, and trapped there, as the water in the recently deposited matrix is displaced by the growing mineral crystals.
Protein Matrix
The protein matrix of bone, as for tendons, ligaments, and dermis, consists predominantly of collagen, which comprises approximately 90% of the organic matrix. For bone, the collagen is type I. Collagen is a long, fibrous protein, coiled as a triple helix. For the molecules of the protein
to coil tightly, no side chains can project from the peptide backbone on the side facing inward. Hence, every third amino acid in the body of the collagen molecule is glycine, which has no side chain. However, projecting outward are the side chains of various other amino acids, such as lysine, which allow the posttranslational formation of tight, covalent bonds between collagen fibers. This cross-linking helps to prevent fibers from sliding along one another when bone is stressed along the axis of the fibers.
to coil tightly, no side chains can project from the peptide backbone on the side facing inward. Hence, every third amino acid in the body of the collagen molecule is glycine, which has no side chain. However, projecting outward are the side chains of various other amino acids, such as lysine, which allow the posttranslational formation of tight, covalent bonds between collagen fibers. This cross-linking helps to prevent fibers from sliding along one another when bone is stressed along the axis of the fibers.
Noncollagenous Matrix Proteins
Noncollagenous proteins make up approximately 10% of the organic matrix of bone (1). These proteins include a family of proteins in which glutamic acid residues are carboxylated in the γ position, called gla-proteins, the best studied of which is osteocalcin (or bone gla-protein), which comprises approximately 1.5% of the matrix proteins. Other gla-proteins include osteonectin, fibronectin, matrix gla-protein, osteopontin, and bone sialoprotein. The functions of these many constituents are not entirely clear. Some doubtless serve as chemoattractants for osteoclasts or as points of osteoclast attachment, whereas others stimulate osteoblasts to lay down new bone. Because of these properties of the matrix proteins, bone seems to contain some of the chemical signals for its own remodeling (see later).
The shape and three-dimensional structure of bone are determined by its protein matrix. A bone that has been completely demineralized in the laboratory (by soaking in ethylenediaminetetraacetic or other acid) looks entirely normal; and when sectioned, stained, and examined under a microscope, it reveals all the fine structure of bone. In fact, prior demineralization has been the traditional first step in studying bone histologically (because mineralized bone tends to damage the microtome knives used by histologists to make their sections).
BONE CELLS AND THEIR FUNCTIONS
The four principal bone cells are lining cells, osteoblasts, osteoclasts, and osteocytes. They are responsible both for maintaining the mechanical properties of bone and mediating the calcium homeostatic function of bone.
Lining cells are flat, fibrocyte-like cells covering free surfaces of bone. They are most probably derived from, or closely related to, the osteoblast cell line. They form a membrane that completely covers free bone surfaces and insulates bone from the cells and hormones in the general circulation. They demarcate a virtual compartment between the lining cells on one side and mature bone on the other. This compartment is continuous with the space in canaliculi surrounding osteocyte processes and may well have different ionic composition from that of the ECF located outside, that is, between the lining cells and the capillaries of bone. It is possible that lining cells, by adjusting ion fluxes between the ECF and the bone compartment, may contribute to the maintenance of calcium ion concentrations in the ECF.
Osteoblasts are derived from marrow stromal cells; they are the cells that lay down bone, first by synthesizing, depositing, and orienting the fibrous proteins of the matrix, and then by initiating changes that render the matrix capable of mineralization. Osteoblasts deposit this matrix between and beneath themselves on a preexisting bone surface, thereby pushing themselves backward as they add new bone.
Bone matrix, when freshly deposited, consists of about half protein and half water and is not immediately mineralizable, just as the similar collagen-based structures, tendon and ligament, do not normally calcify. So the osteoblast still has more work to do after forming and depositing the matrix. The details of the process are not completely clear, but they involve secretion of proteins by the osteoblast into the matrix that it had just previously laid down. These somehow help to create a three-dimensional configuration that allows calcium and phosphate ions in the ECF to arrange themselves in the apatite crystal habitus. Osteoblasts also secrete an enzyme called alkaline phosphatase that hydrolyzes various phosphate compounds in the local environment, thereby increasing phosphate ion concentration at the mineralizing site and at the same time removing natural crystal inhibitors (e.g., pyrophosphate). Finally, as mineral is deposited, it displaces the water of the original matrix. The apatite crystals that form are spindle shaped and are oriented parallel to and between the collagen fibers.
Osteoclasts are derived from the monocyte-macrophage line of cells, are usually multinucleated, and are the cells that resorb bone. They do this first by attaching firmly to a microscopic bony surface and then walling off a small region of that surface. The attachment involves linkage between proteins called integrins in the cell membrane of the osteoclast with proteins in the bone matrix, such as osteopontin, that exhibit a particular amino acid sequence (RGD, i.e., arginine-glycine-asparagine). Once firmly attached, osteoclasts secrete acid and proteolytic enzymes into this confined space. These dissolve the mineral and digest the matrix. The osteoclasts then release the breakdown products into the ECF around the resorption site, whence they are carried away by the circulating blood. After working for a short period of time (measured in days), the osteoclasts undergo programmed cell death (apoptosis), leaving their excavation to be refilled by osteoblasts. The calcium they dissolve from bone mineral seems to trigger or augment this apoptotic process, as osteoclasts blocked from producing acid accumulate on bone surfaces and have longer life spans. When examined histologically in sections of bone, these processes necessarily appear localized. However, the activity is more typically a “project,” moving along a bony surface, with osteoclastic work preceding and the osteoblastic formation filling in behind.
The calcium and phosphorus released into the bloodstream at a resorption site will usually be used to mineralize
remodeling sites elsewhere in the skeleton, currently in their formation phase. However, the protein fragments are metabolized or excreted. Some of the amino acids released in collagen degradation reenter the body’s amino acid pool and can be reused for protein synthesis elsewhere. However, those that have undergone posttrans-lational modification (e.g., proline to hydroxyproline and the amino acids involved in collagen cross-linking) cannot be reused. For this reason, bone remodeling requires a continuing supply of fresh dietary protein.
remodeling sites elsewhere in the skeleton, currently in their formation phase. However, the protein fragments are metabolized or excreted. Some of the amino acids released in collagen degradation reenter the body’s amino acid pool and can be reused for protein synthesis elsewhere. However, those that have undergone posttrans-lational modification (e.g., proline to hydroxyproline and the amino acids involved in collagen cross-linking) cannot be reused. For this reason, bone remodeling requires a continuing supply of fresh dietary protein.
Osteocytes are osteoblasts that have stopped matrix synthesis and have become embedded in bone as the other bone-forming cells around them continue to add new layers of matrix. Osteocytes are responsible for monitoring the amount of strain (bending) that occurs in their domains when bone is mechanically loaded and for reporting that information to lining cells on nearby anatomic bone surfaces, which may then initiate local bone remodeling projects. One of the ways they do this is by secreting a hormone, sclerostin, that reduces osteoblast activity (see below). The full extent of their function is not known, but it is clear that bone with dead osteocytes (from whatever cause) is often excessively fragile.
The activity of these bone cells is influenced by a large number of both systemic and local hormonal agents. Additionally, the cells influence the activity of one another. Table 89.1 lists a few of the many agents influencing osteoblasts and osteoclasts (see the later section on revision of bony material). This is a rapidly developing field of investigation, and much is still to be learned. The osteoblasts, or cells of the osteoblast lineage, occupy a central position, not only in forming bone but also in processing systemic signals to the bone remodeling apparatus (see the later section on revision of bony material). Thus, although parathyroid hormone (PTH) is responsible for stimulating bone resorption, no PTH receptors are present on the osteoclast. Rather, they are found on osteoblasts (and related cells) that, in response to PTH binding, release or express on their surfaces agents (e.g., RANK ligand [RANKL]) that stimulate osteoclast activity. By contrast, the osteoclasts do possess calcitonin (CT) receptors and are thus able to respond very rapidly to the antiresorptive signal provided by secreted CT.
TABLE 89.1 HUMORAL FACTORS ACTING ON BONE CELLS | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
BONE ARCHITECTURE
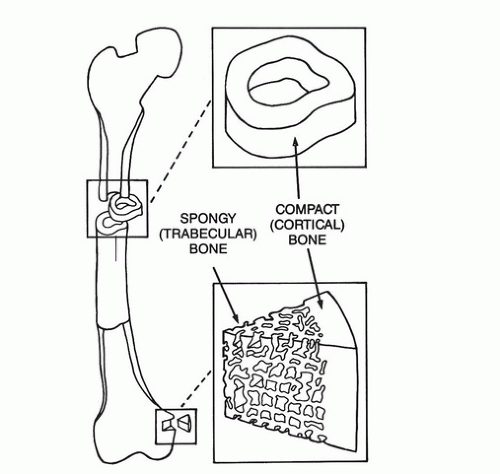
Bone consists of a dense outer shell, or cortex, and an internal, chambered system of interconnected plates, rods, and spicules called cancellous or trabecular bone (Fig. 89.1). In the shafts of the long bones, the cortical component predominates, creating a hollow tube, whereas nearer the joints, the cortex becomes thinner and the interior is made up of an extensive latticework of cancellous bone. Bones such as the vertebrae, pelvis, sternum, and shoulder blades possess a thin outer rind of cortex and a more or less even distribution of cancellous bone on the inside. The internal, three-dimensional architecture of cancellous bone is arranged along the lines of force that a particular bone experiences and hence provides maximum structural strength with minimum material.
The proportions both of mineral and matrix and of calcium and phosphorus are essentially identical in cortical and cancellous bone. Sometimes the issue has been confused in the literature because of the difficulty of removing adherent marrow elements from cancellous bone samples before chemical analysis. Fundamentally, however, bone is bone. On the other hand, cancellous bone turns over (remodels) much more rapidly than cortical bone. This is partly because of the much greater surface area of cancellous bone. (Remodeling always starts from a microanatomic bone surface and burrows into the bony material. See the later section on revision of bony material.) It is also partly the result of the generally greater contact with hemopoietic marrow in cancellous bone.
In fact, the partition of remodeling between bone with and bone without red marrow is probably more important than the partition between cortical and trabecular bone.
In fact, the partition of remodeling between bone with and bone without red marrow is probably more important than the partition between cortical and trabecular bone.
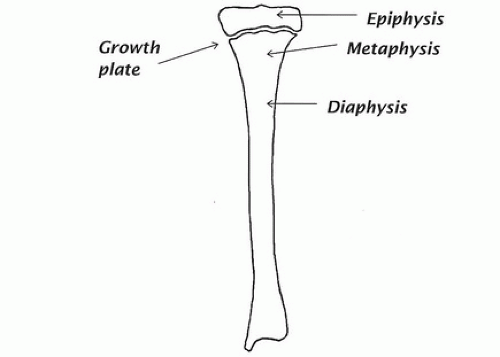
The end segments of bones are called epiphyses (Fig. 89.2). The shafts of long bones are called diaphyses, and the flared portion of the shaft merging with the region of the growth plate is called a metaphysis. The lining cells on the outside of the bone form a tough sheet or membrane, called the periosteum, whereas the cells on the inside surfaces of both cortical and trabecular bone are called the endosteum.
The spaces between the trabecular plates and spicules are filled with bone marrow. Early in life, much of that marrow is hemopoietic, but later the blood-producing marrow is confined to the bones of the trunk, and the peripheral skeletal marrow spaces are filled mostly with fat.
In dense cortical bone, remodeling over the years produces a series of internal structures called osteons or Haversian systems, in which concentric cylindric layers of bone are laid down along the course of a capillary. These are produced by the usual remodeling process (see later, in the section on revision of bony material). First, a tubular hole is created in the bone by osteoclastic resorption; then, it is filled in by successive waves of osteoblasts moving from the outside inward.
At their ends, where bones meet one another in a joint, the bony surface is covered with a layer of cartilage rather than with periosteum. In health, this cartilage, is highly hydrated and is lubricated by synovial fluid held there by a tough connective tissue sac called the joint capsule. This arrangement ensures that the bones move on one another smoothly.
BONE DEVELOPMENT
In utero, most bones are formed first as cartilage models, which are gradually replaced by bone. In this process, blood vessels invade the cartilage, calcification ensues, and the calcified cartilage is then removed by osteoclasts and replaced by bone laid down by osteoblasts. In infancy and childhood, bone growth and development follow a similar pattern. However, to accommodate growth, most bones possess one or more plates of cartilage perpendicular to the main axis of growth, separating, for example, the bone at the ends of a long bone from the bone of the shaft. This structure, called a growth plate (see Fig. 89.2), consists of rapidly proliferating cartilage cells that push the ends of the bones away from the shafts as they multiply. Blood vessels invade these columns of proliferating cartilage cells from the shaft side and initiate calcification of the cartilage and bony replacement in a process similar to what occurs in utero. Meanwhile, the ends of the bone are being pushed still further away; thus there continues to be an anatomic and temporal sequence consisting of growth plate, proliferating cartilage, calcifying cartilage, and bony replacement, progressing down the metaphysis from the epiphyses toward the diaphysis.
Growth stops when the ossification process catches up with the formation of new cartilage at the growth plate and bony bridges develop across the growth plate, thus firmly anchoring the ends to the shaft of the bone. This closure process is initiated by the high levels of estrogen produced at puberty in both girls and boys. During the time when growth in length is occurring, the process of modeling (see later) shapes the outsides of the bones to keep them in proper proportion with the growth in length. At midshaft, this typically involves periosteal new bone formation and endosteal resorption, whereas at the metaphysis, it is the other way about, with periosteal resorption and endosteal formation.
REVISION OF BONY MATERIAL
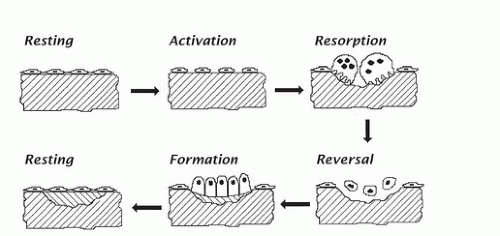
Although the intercellular bony material, which makes up 95% to 98% of the volume of bone, is nonliving, nevertheless, it is capable of being revised and replaced, just as are soft tissues, whose cellular constituents are turning over constantly and invisibly. In bone, the revision process occurs at discrete locations and is readily visualizable microscopically. The process normally follows a stereotyped sequence of activation first, then resorption, then reversal, and finally formation. This sequence is shown diagrammatically in Figure 89.3.
In the activation step, lining cells on a bone surface retract, exposing the bony substance directly to the circulating blood. Mineralized bone serves as a chemoat-tractant for osteoclast precursors, which migrate to the exposed site, coalesce, attach to bone as osteoclasts, and begin to erode into the bone. After removing a suitable volume of bone, osteoclasts undergo apoptosis and disappear from the site. (But, as noted earlier, the process drifts along a bony surface, and new osteoclasts are recruited to keep the process going.) A reversal phase then follows. Osteoblasts move in and begin to replace the bone removed from the cavity. They orient the collagen fibers in parallel arrays, layer by layer, often alternating direction of the fibers every few micrometers. In this way, they build what is termed lamellar bone, which is similar in a sense to plywood (in which the grain of the wood runs in different directions in different layers). In the adult, osteoblasts advance at a rate of approximately 0.5 μm/day, and mineralization lags approximately 10 days behind the advancing matrix deposition. Some of the osteoblasts, as already noted, stay behind in cavities within the bone and become osteocytes.
When this revision process is finished at a particular site, the remaining surface osteoblasts become quiescent, flatten out, and turn into lining cells, effectively sealing the new bone surface until, at some later time, a new remodeling project is initiated locally. In healthy adults, this sequence takes approximately 3 months at any given site from start to finish. The resorptive phase takes approximately 2 to 3 weeks, and formation takes 2 to 3 months. The process is faster in infants and small children and is typically slower in elderly persons.
The coordination of this remodeling activity is mediated by a still-growing list of cell-to-cell signals and interactions that both stimulate and inhibit the forming and resorbing processes. Briefly, cells of the osteoblast line secrete two substances: RANKL and osteoprotegerin. The former initiates osteoclast cell formation and differentiation and is necessary for the continued function of osteoclastic resorption. Osteoprotegerin complexes with RANKL and prevents its docking with RANK on the osteoclast surface. Thus osteoblasts and narrow stromal cells orchestrate bone resorption. At the same time, osteoclasts themselves secrete an as yet unidentified factor that stimulates osteoblastic bone formation. Finally, osteocytes also enter into this control matrix by secreting sclerostin, a glycoprotein that blocks the stimulation of osteoblastic new bone formation. The functionality of this last interaction is perhaps easiest to grasp: Osteocytes cannot function if they are buried so deeply in new forming bone as to have no access to a blood supply. Thus sclerostin is hypothesized to be secreted by osteocytes at the limit of the supply chain, effectively stopping further bone formation at any particular site.
The process just described is technically called remodeling. One of its principal purposes is to replace damaged volumes of bone with fresh new material. During growth, however, and indeed at any time when the shape of the bone is being revised, the resorptive and formative processes occur, not at the same site, but in different parts of the bone. For example, the small shafts of the long bones of a child are enlarged to adult size by osteoblastic formation on the periosteal surface and by corresponding osteoclastic resorption on the endosteal surface. This process is technically called modeling and is similar to remodeling in most respects, except the new bone is laid down at a location different from the site of resorption.
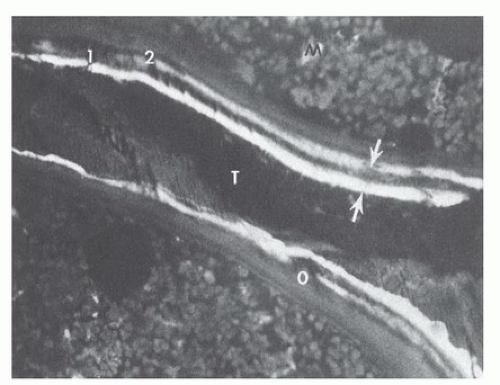
One of the ways in which this revision process can be readily visualized is by the administration of carefully timed doses of a fluorescent compound such as one of the tetracycline antibiotics. The molecules of these markers bind to mineralizing bone sites and are trapped as the site is buried in layers of new bone. The markers become readily visible, appearing like tree rings, when undecalcified bone biopsy specimens are viewed microscopically under ultraviolet illumination (Fig. 89.4).
BONE FUNCTIONS
Bone serves two distinct functions: the provision of mechanical rigidity and stiffness to our bodies; and the provision of a homeostatic buffer, particularly to help our bodies maintain a constant level of calcium in the circulating body fluids and to provide a reserve supply of phosphorus. The mechanical function is necessary so that we can resist gravity and move about on dry land. (Strictly speaking, a rigid skeleton would not be necessary in a fully buoyant medium such as water. For example, some originally bony fish, such as the sturgeon, have lost nearly all of their skeletons over the millennia of evolution. Still their mechanical function is maintained.) The
homeostatic function of bone is the older of the two, from the standpoint of evolution, and is in a sense the more fundamental, because the body will sacrifice the structural function before it will risk losing the homeostatic one. In other words, the body will weaken the bone structurally to maintain the calcium levels of the blood and ECF.
homeostatic function of bone is the older of the two, from the standpoint of evolution, and is in a sense the more fundamental, because the body will sacrifice the structural function before it will risk losing the homeostatic one. In other words, the body will weaken the bone structurally to maintain the calcium levels of the blood and ECF.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree