
II. Transfusion Therapy for Bleeding. Critical bleeding in the perioperative setting requires volume replacement (crystalloid, colloid, red blood cells [RBCs]) (none provide coagulation factors or platelets and thus their use can exacerbate coagulopathy). Severe bleeding requires use of fresh frozen plasma (FFP), platelets, cryoprecipitate, and factor concentrates (e.g., fibrinogen and prothrombin complex concentrates) to restore circulating levels of hemostatic factors. Following massive transfusion therapy, hypothermia and acidosis (temperature and pH) must be monitored and corrected during any ongoing transfusion.
A. Red Blood Cells
1. There is no single minimum acceptable hemoglobin level that can be applied to all patients when deciding when to transfuse RBCs. With acute anemia, compensatory mechanisms that increase cardiac output and improve oxygen transport depend on the patient’s cardiovascular reserve. In surgical patients with heart failure and/or flow-restricting lesions, compensation during acute anemia may be limited.
2. The decision to transfuse must include multiple factors (intravascular volume, whether the patient is actively bleeding, and the need for improvement in oxygen transport).
a. The American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion noted in its recommendations that transfusion of RBCs should usually be administered when the hemoglobin concentration is low (e.g., <6 g/dL in a young, healthy patient), especially when the anemia is acute.
b. RBCs are usually unnecessary when the hemoglobin concentration is more than 10 g/dL.
c. Hemoglobin triggers for transfusion are not to be taken as absolute; patients with significant cardiac disease should be transfused if signs or symptoms of inadequate myocardial oxygenation appear.
B. RBC Storage Lesions
1. RBCs develop a complex series of biochemical and metabolic changes during storage in the blood bank. As the blood ages, RBCs undergo shape changes with increased fragility.
2. Because of increased red cell–endothelial cell interaction, bioreactive lipids and other substances are released that may initiate inflammatory responses leading to TRALI.
C. RBC Storage and Tissue Oxygenation Parameters
1. Transfusion of RBCs is used therapeutically to increase the oxygen-carrying capacity of blood and thereby improve oxygen delivery to tissues.
2. The true impact of the age of stored RBC on patient outcomes remains in question, but an answer should emerge from these ongoing clinical trials.
D. Plasma/fresh frozen plasma (FFP) are used to replace volume and coagulation factors during massive transfusion (Table 28-3).
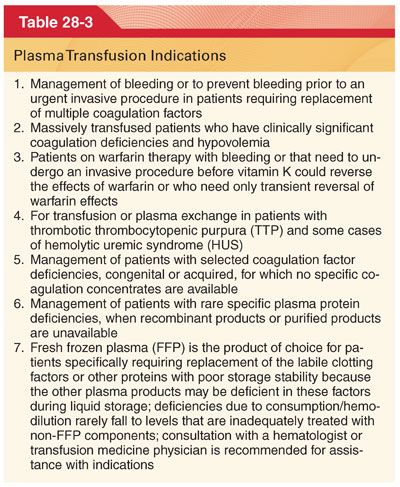
1. FFP is used for treating bleeding because of coagulopathies that are associated with a prolongation of either the activated partial thromboplastin time or prothrombin time/international normalized ratio greater than 1.5 times normal.
2. When FFP is indicated, it should be administered in a dose calculated to achieve a minimum of 30% of plasma factor concentration (10 to 15 mL/kg of FFP will generally result in a rise of most coagulation proteins by 25% to 30%).
3. Plasma is overused in surgery, most often because of the empirical nature of transfusion therapy.
a. The most common cause of bleeding after surgery is platelet dysfunction.
b. The prothrombin time and partial thromboplastin times, which are widely used to evaluate bleeding, have never been demonstrated to accurately reflect the cause of bleeding in surgical patients.
4. Plasma transfusions, like all blood products, have the potential for adverse effects (transfusion-associated circulatory overload, TRALI).
E. Solvent treated plasma is human pooled plasma that has been solvent/detergent (S/D) treated (kills certain viruses and minimizes the risk of serious virus transmission) and is thought to reduce the risk of TRALI. This product is indicated for replacement of multiple coagulation (administration is based on ABO blood group compatibility).
F. Cryoprecipitate contains therapeutic amounts of factor VIII:C, factor XIII, von Willebrand factor, and fibrinogen.
1. Cryoprecipitate is used not only to increase fibrinogen levels depleted because of massive hemorrhage or coagulopathy but also for the treatment of congenital or acquired factor XIII deficiency.
2. One unit of cryoprecipitate per 10 kg body weight increases plasma fibrinogen by roughly 50 to 70 mg/dL in the absence of continuing consumption or massive bleeding. The minimum hemostatic level of fibrinogen is traditionally suggested to be around 100 mg/dL (normal fibrinogen levels are 200 mg/dL and higher).
3. Because cryoprecipitate does not contain factor V, it should not be the sole replacement therapy for disseminated intravascular coagulopathy (DIC).
4. Cryoprecipitate has been withdrawn from many European countries due to safety concerns, primarily the transmission of pathogens (instead, commercial fibrinogen preparations are available for fibrinogen replacement therapy).
G. Platelet Concentrates
1. Platelets that are used clinically are either pooled random-donor platelet concentrates or single-donor apheresis and can be stored for up to 5 days.
2. In medical patients, a platelet count of 10,000/μL is a typical threshold for prophylactic platelet transfusion (normal platelet count ranges from 150,000 to 400,000 platelets per μL). The platelet count for therapeutic transfusions to control or prevent bleeding with trauma or surgical procedures requires a higher transfusion trigger of 100,000/μL for neurosurgical procedures and between 50,000/μL and 100,000/μL for other invasive procedures or trauma.
3. There remains significant risk of bacterial infection with platelet administration because they are stored at 22°C rather than at the 4°C storage used for red cell storage.
H. Alloimmunization. An immunocompetent recipient often develops variable immune responses to the transfused agents that include graft versus host disease. Multiple other antigens that are not routinely crossmatched for platelets may be responsible for alloimmunization.
I. Leukoreduction. Leukoreduced platelet and RBC products decrease the risk of alloimmunization. Cytomegalovirus transmission is reduced by reducing leukocyte burden, and as a result, there is also a reduction in febrile transfusion reactions.
III. Graft versus host disease (GVHD) is a potentially fatal complication that occurs more commonly after a bone marrow or stem cell transplant, or following platelet transfusions where viable white cells from the donor regard the recipient’s body as foreign and create acute inflammatory responses and tissue and organ injury by attacking the recipient’s body (γ-irradiation is performed for prevention of GVHD).
IV. Indications for Platelet Transfusions and Transfusion Triggers
A. In medical patients, a platelet transfusion trigger of approximately 10,000 platelets/μL in efforts to prevent bleeding is often described (yet data and prospective studies to evaluate the effects of platelet dose on hemostasis and rates of platelet use overall for perioperative management are often based on consensus guidelines rather than clinical studies).
B. There are three important areas of controversy regarding the use of platelet transfusions without active bleeding. First, the optimal prophylactic platelet dose to prevent thrombocytopenic bleeding even in medical patients is not well known. Second, the exact platelet count threshold that requires transfusion of platelets is not known. Finally, whether prophylactic platelet transfusions are superior to therapeutic platelet transfusions in surgical patients is not known.
1. In most surgical patients, there is little data to support prophylactic platelet transfusions; the exceptions are massive transfusion coagulopathy and certain closed procedures where bleeding may be highly problematic such as intracranial hemorrhage.
2. Dilutional thrombocytopenia often occurs as an early manifestation of massive transfusion.
C. Platelet Counts for Surgery and Invasive Procedures
1. For surgery or following trauma, expert recommendations suggest that a platelet count of greater than or equal to 50,000/μL be maintained (little data to support these recommendations).
2. In neurosurgical patients or patients with intracerebral bleeding and for neurosurgical procedures, expert recommendations suggest that platelet counts should be maintained at greater than 100,000/μL.
3. With platelet counts between 50,000 and 100,000/μL, clinical decisions to transfuse platelets should be based on the type of surgery, trauma, rates of bleeding, risk of bleeding, use of platelet inhibitors, and other potential coagulation abnormalities.
4. If platelet dysfunction is present in the face of trauma or surgery, platelet transfusions may be necessary, even in the presence of a normal platelet count.
5. ABO Compatibility
a. Red cell antigens are expressed on platelets, and ABO-incompatible platelets have reduced posttransfusion platelet count recoveries but normal platelet survival.
b. Patients who receive ABO-incompatible platelets become refractory to additional platelet transfusions at a higher rate than the ABO-compatible recipients because sensitization produces anti–human leukocyte antigen (HLA) and platelet-specific alloantibodies.
V. Purified Protein Concentrates
A. Fibrinogen Concentrates. Fibrinogen is a critical clotting protein (cryoprecipitate is routinely administered as the source of fibrinogen). Fibrinogen concentrate administration in patients with hypofibrinogenemia and disseminated intravascular coagulation should be avoided.
B. Prothrombin complex concentrates are recommended for reversal in patients with life-threatening bleeding and an increased international normalized ratio when urgent reversal is required (warfarin reversal in the United States occurs with FFP).
C. von Willebrand Factor is indicated in adult and pediatric patients with von Willebrand’s disease for (a) treatment of spontaneous and trauma-induced bleeding episodes and (b) prevention of excessive bleeding during and after surgery.
VI. Hereditary Angioedema and C1 Esterase Inhibitor Concentrates
A. Hereditary angioedema (HAE) is a life-threatening disease resulting from the absence or genetic mutation of a complement component inhibitor called C1 esterase inhibitor (C1 INH).
B. Angioedema produces increased permeability of submucosal or subcutaneous capillaries and postcapillary venules leading to plasma extravasation and subsequent swelling of critical airway structures.
VII. Adverse Effects of Transfusions. The risks of allogeneic transfusion extend beyond viral transmission, and include allergy, alloimmunization, anaphylaxis, bacterial sepsis, graft versus host disease, TRALI, transfusion-related acute circulatory overload, renal failure, volume overload, and immunosuppression (Table 28-4).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


