14 Blood disorders
Blood diseases cover a wide spectrum of illnesses, ranging from common anaemias to rare conditions such as leukaemias and congenital coagulation disorders. Haematological change may occur as a consequence of disease affecting any system, and measurement of haematological parameters is an important part of routine clinical assessment.
PRESENTING PROBLEMS
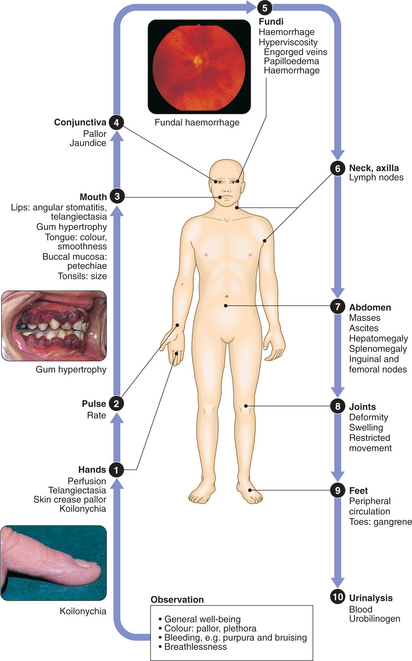
CLINICAL EXAMINATION IN BLOOD DISORDERS
The diagnosis of anaemia not only includes an assessment of its clinical severity but must also define the underlying cause. This rests on history and examination, FBC and blood film, and further investigations.
HIGH HAEMOGLOBIN
A Hb level > the upper limit of normal (adult females 165 g/l, adult males 180 g/l) may be due to an increase in the number of red blood cells (true polycythaemia) or a reduction in the plasma volume (relative or apparent polycythaemia); these can be distinguished by isotope measures of red cell mass.
True polycythaemia: Caused by increased erythropoiesis in the bone marrow. This may be due to a primary increase in marrow activity (polycythaemia rubra vera, PRV) or secondary to increased erythropoietin (Epo) production, either as a consequence of chronic hypoxaemia or because of inappropriate erythropoietin secretion (e.g. lung or renal disorders) (Box 14.1). History and examination will provide clues to the aetiology of true polycythaemia. Those with PRV may have arterial thromboses, pruritus, gout due to high red cell turnover and hepatosplenomegaly. The cardiovascular and respiratory systems should be assessed for causes of hypoxaemia, and further investigations to exclude inappropriate erythropoietin secretion should be performed.
14.1 CAUSES OF TRUE POLYCYTHAEMIA ![]()
| Aetiology | Examples | |
|---|---|---|
| Primary | Myeloproliferative disorder | Polycythaemia rubra vera (PRV) |
| Secondary |
LYMPHADENOPATHY
Enlarged lymph nodes may be an indicator of haematological disease but can also occur in reaction to infection or inflammation (Box 14.2). Reactive nodes usually expand rapidly and are painful, whereas those due to haematological disease are frequently painless and may be generalised. Initial investigations in lymphadenopathy should include:
14.2 CAUSES OF LYMPHADENOPATHY ![]()
| Infective | |
| Neoplastic | |
| Inflammatory | |
| Miscellaneous | Amyloidosis |
If the findings suggest malignancy, lymph node biopsy is required.
SPLENOMEGALY
Causes of splenic enlargement are listed in Box 14.3.
| Congestive | |
| Infective | |
| Inflammatory/granulomatous disorders | e.g. Felty’s syndrome, SLE, sarcoidosis |
| Haematological | Myeloproliferative disorders, e.g. chronic myeloid leukaemia, myelofibrosis, PRV, essential thrombocythaemia |
BLEEDING
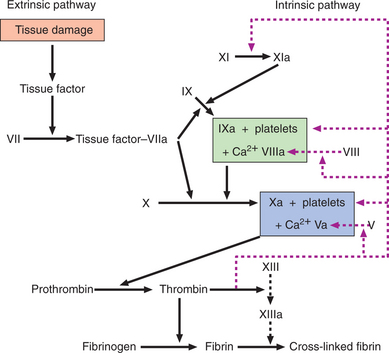
Normal haemostasis
Haemostasis depends upon interactions between the vessel wall, platelets and clotting factors. In the initial primary phase, the damaged vessel constricts and platelets aggregate to form a plug. This is followed by activation of the coagulation cascade, resulting in the formation of a cross-linked fibrin clot (Fig. 14.1). Clotting factors are synthesised by the liver and several are dependent on vitamin K for their activation; activated factors are designated by the suffix ‘a’. The extrinsic pathway is the main physiological mechanism in vivo.
History
THROMBOCYTOPENIA (LOW PLATELETS)
Causes of thrombocytopenia are listed in Box 14.4. Purpura, bruising and spontaneous oral, nasal, GI or GU bleeding may occur but not usually until the platelet count falls below 30 × 109/l. Blood film may be diagnostic (e.g. acute leukaemia). Bone marrow examination may reveal:
14.4 CAUSES OF THROMBOCYTOPENIA ![]()
| Marrow disorders | |
| Increased platelet consumption |
VENOUS THROMBOSIS
Deep venous thrombosis (DVT) must be excluded in patients presenting with unilateral leg-swelling. Other causes include a calf haematoma, cellulitis and a ruptured Baker’s cyst (which normally occurs in rheumatoid arthritis of the knee). Bilateral leg-swelling may result from extensive proximal DVT extending into the inferior vena cava, impaired venous or lymphatic return due to obstruction in the pelvis, right-sided heart failure and hypoalbuminaemia.
Using the patient’s symptoms, the probability of DVT can be established using the Well’s score (Box 14.5). Those with a medium or high risk should undergo further investigation.
14.5 PROCEDURE FOR PREDICTING THE PRE-TEST PROBABILITY OF DVT ![]()
| Clinical characteristic | Score |
|---|---|
| Active cancer (patient receiving treatment for cancer within the previous 6 mths or currently receiving palliative treatment) | 1 |
| Paralysis, paresis or recent plaster immobilisation of the lower extremities | 1 |
| Recently bedridden for 3 days or more, or major surgery within the previous 12 wks requiring general or regional anaesthesia | 1 |
| Localised tenderness along the distribution of the deep venous system | 1 |
| Entire leg swollen | 1 |
| Calf swelling at least 3 cm larger than that on the asymptomatic side (measured 10 cm below tibial tuberosity) | 1 |
| Pitting oedema confined to the symptomatic leg | 1 |
| Collateral superficial veins (non-varicose) | 1 |
| Previously documented DVT | 1 |
| Alternative diagnosis at least as likely as DVT | − 2 |
| Clinical probability | Total score |
| DVT unlikely | <2 |
| DVT likely | ≥2 |
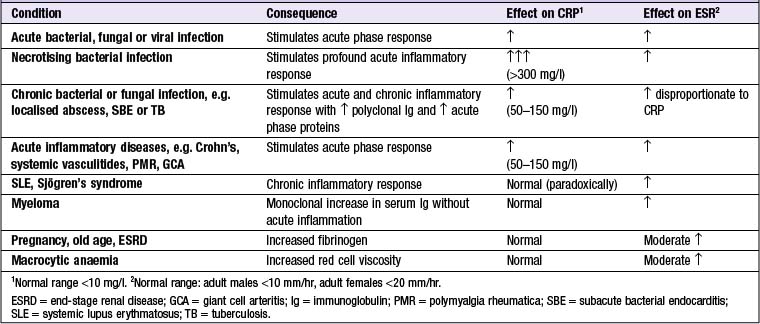
ELEVATED MARKERS OF INFLAMMATION (Box 14.6)
C-REACTIVE PROTEIN (CRP)
CRP is an acute phase reactant which opsonises invading pathogens. Levels of CRP increase within 6 hrs of an inflammatory stimulus. The short plasma half-life of CRP (19 hrs) means it can be used to monitor disease activity. Some diseases (e.g. SLE, systemic sclerosis, ulcerative colitis and leukaemia) are associated with only minor elevations of CRP despite the presence of inflammation. Intercurrent infection, however, does provoke a significant CRP response in these conditions.
ERYTHROCYTE SEDIMENTATION RATE (ESR)
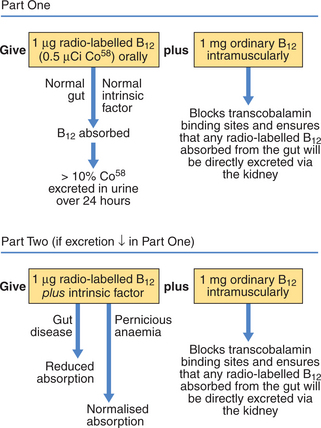
Investigations
BLOOD PRODUCTS AND TRANSFUSION
BLOOD PRODUCTS
In the UK, in order to minimise the risk of passing on variant Creutzfeldt–Jakob disease (vCJD, p. 674), all blood components are processed to remove white cells, and in addition transfusion recipients are not subsequently accepted as blood donors.
Platelet concentrate: Used to treat and prevent bleeding due to thrombocytopenia.
Fresh frozen plasma (FFP): Used to replace coagulation factors.
RED CELL COMPATIBILITY
There are four different ABO groups, determined by whether or not an individual’s red cells express A or B antigens. Healthy individuals have antibodies directed against the A or B antigens that are not expressed on their own cells (Box 14.7). If red cells of an incompatible ABO group are transfused, the patient’s antibodies bind to the transfused cells leading to red cell haemolysis. This is the main cause of an acute transfusion reaction, and can lead to disseminated intravascular coagulation (DIC), renal failure and death.
14.7 THE ABO SYSTEM ![]()
| Blood group | Red cell A or B antigens | Antibodies in plasma |
|---|---|---|
| O | None | Anti-A and anti-B |
| A | A | Anti-B |
| B | B | Anti-A |
| AB | A and B | None |
About 15% of Caucasians lack the Rhesus D (RhD) red cell antigen (‘Rhesus-negative’). IgG antibodies to RhD-positive red cells are produced if such cells enter the circulation of an RhD-negative individual via fetomaternal haemorrhage during pregnancy. During a subsequent pregnancy with an RhD-positive fetus, these antibodies can cross the placenta and cause haemolytic disease of the newborn and severe neurological damage. Administration of anti-RhD immunoglobulin (anti-D) after delivery blocks the immune response to the RhD antigen and prevents the development of Rhesus antibodies in RhD-negative women.
ADVERSE EFFECTS OF TRANSFUSION
ANAEMIAS
Around 30% of the world population is anaemic; iron deficiency is the cause in half of these.
IRON DEFICIENCY ANAEMIA
Causes of iron deficiency include: