Erythrocytes
Blood is a specialized connective tissue in which cells are suspended in fluid extracellular material called plasma. Propelled mainly by rhythmic contractions of the heart, about 5 L of blood in an average adult moves unidirectionally within the closed circulatory system. The so-called formed elements circulating in the plasma are erythrocytes (red blood cells), leukocytes (white blood cells), and platelets.
When blood leaves the circulatory system, either in a test tube or in the extracellular matrix (ECM) surrounding blood vessels, plasma proteins react with one another to produce a clot, which includes formed elements and a pale yellow liquid called serum. Serum contains growth factors and other proteins released from platelets during clot formation, which confer biological properties very different from those of plasma.
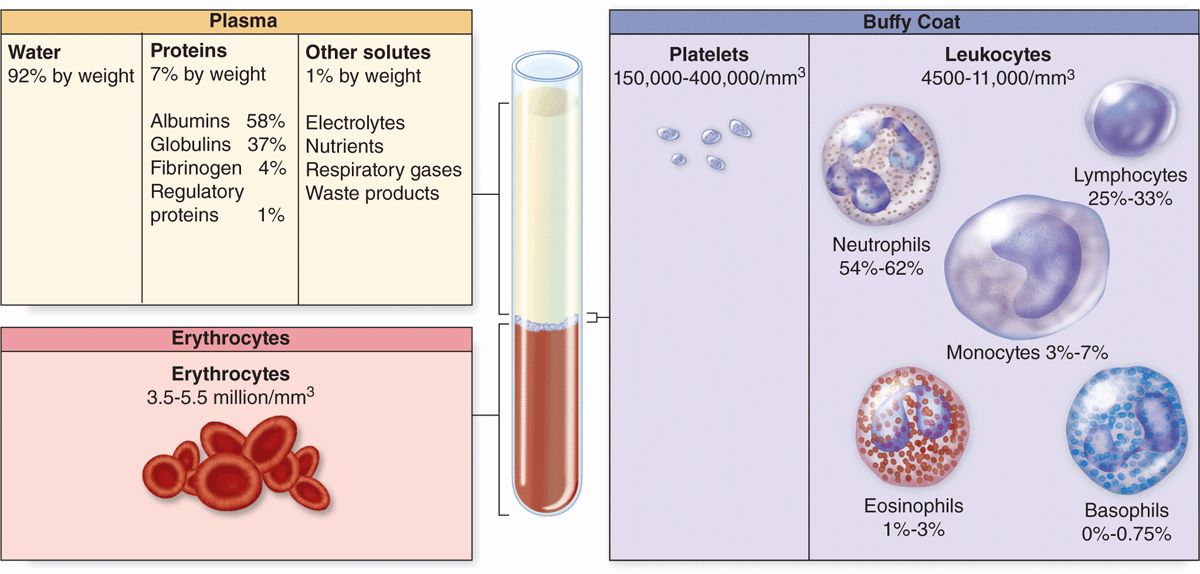
Collected blood in which clotting is prevented by the addition of anticoagulants (eg, heparin or citrate) can be separated by centrifugation into layers that reflect its heterogeneity (Figure 12–1). Erythrocytes make up the sedimented material and their volume, normally about 45% of the total blood volume in healthy adults, is called the hematocrit.
FIGURE 12–1 Composition of whole blood.
The strawcolored, translucent, slightly viscous supernatant comprising 55% at the top half of the centrifugation tube is the plasma. A thin gray-white layer called the buffy coat between the plasma and the hematocrit, about 1% of the volume, consists of leukocytes and platelets, both less dense than erythrocytes.
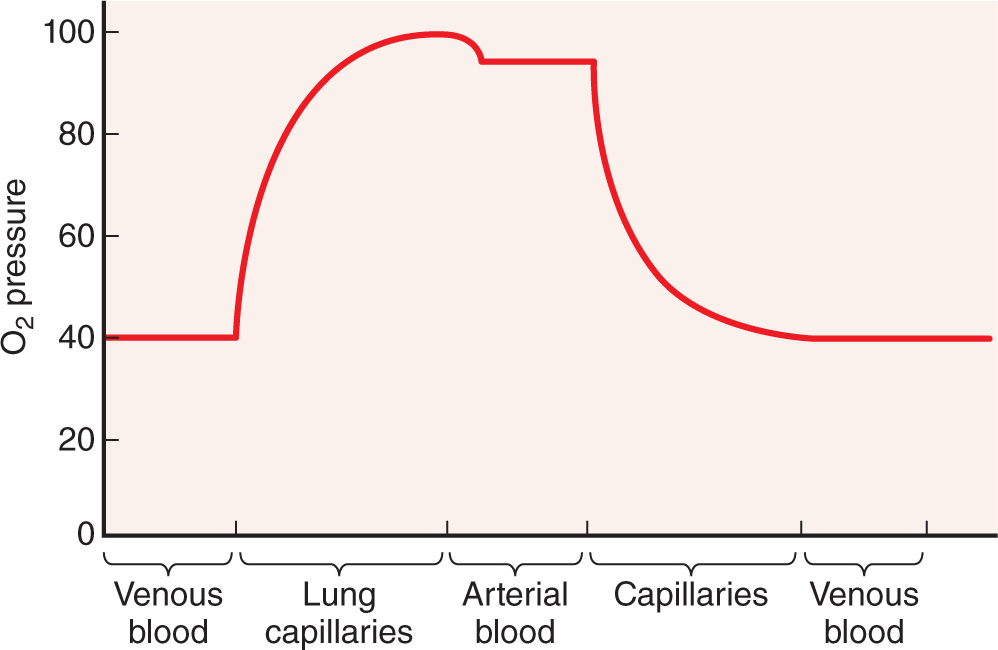
Blood is a distributing vehicle, transporting O2, CO2, metabolites, hormones, and other substances to cells throughout the body. O2 is bound mainly to hemoglobin in erythrocytes and is much more abundant in arterial than venous blood (Figure 12–2), while CO2 is carried in solution as CO2 or HCO3–, in addition to being hemoglobin-bound. Nutrients are distributed from their sites of synthesis or absorption in the gut, while metabolic residues are collected from cells all over the body and removed from the blood by the excretory organs. Hormone distribution in blood permits the exchange of chemical messages between distant organs regulating normal organ function. Blood also participates in heat distribution, the regulation of body temperature, and the maintenance of acid-base and osmotic balance.
FIGURE 12–2 Blood O2 content in each type of blood vessel.
Leukocytes have diversified functions and are one of the body’s chief defenses against infection. These cells are generally spherical and inactive while suspended in circulating blood, but, when called to sites of infection or inflammation, they cross the wall of venules, become motile and migrate into the tissues, and display their defensive capabilities.
COMPOSITION OF PLASMA
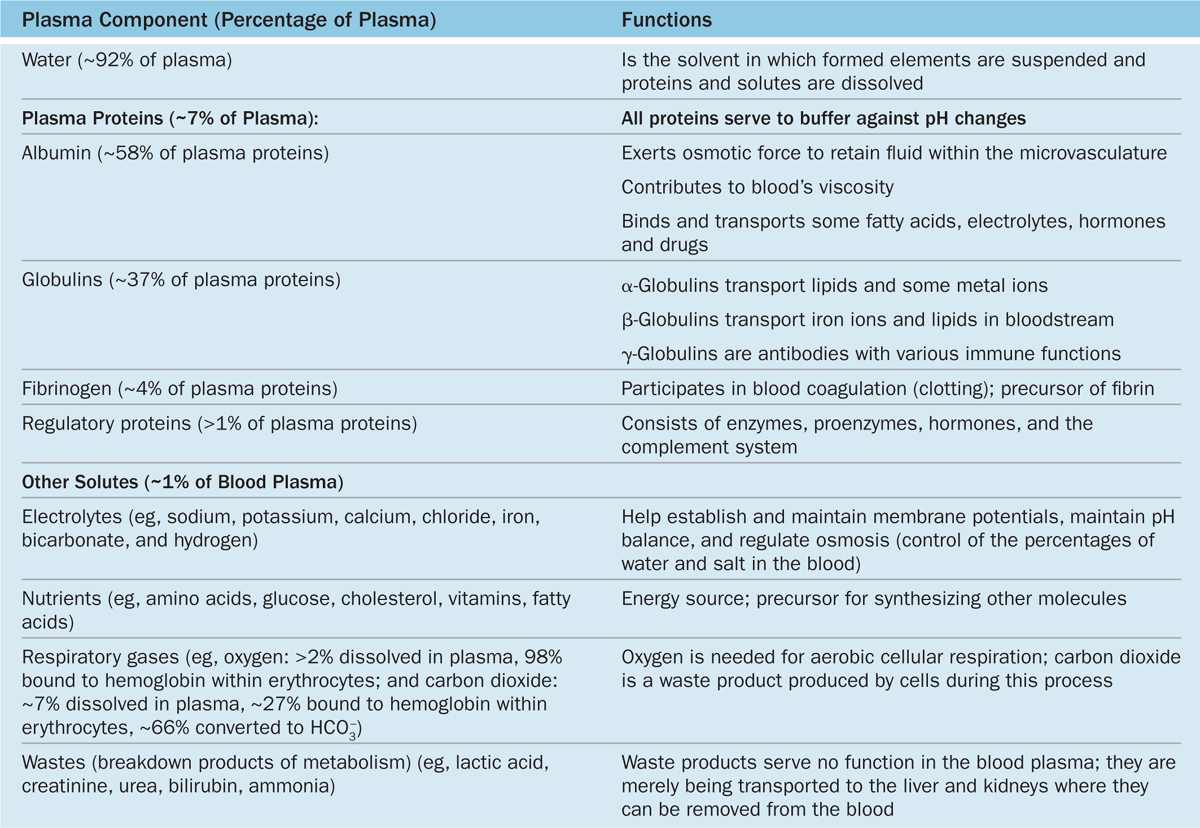
Plasma is an aqueous solution, pH 7.4, containing substances of low or high molecular weight that make up 7% of its volume. As summarized in Table 12–1, the dissolved components are mostly plasma proteins, but they also include nutrients, respiratory gases, nitrogenous waste products, hormones, and inorganic ions, collectively called electrolytes. Through the capillary walls, the low-molecular-weight components of plasma are in equilibrium with the interstitial fluid of the tissues. The composition of plasma is usually an indicator of the mean composition of the extracellular fluids in tissues.
TABLE 12–1 The composition of blood plasma.
The major plasma proteins include the following:
 Albumin, the most abundant plasma protein, is made in the liver and serves primarily to maintain the osmotic pressure of the blood.
Albumin, the most abundant plasma protein, is made in the liver and serves primarily to maintain the osmotic pressure of the blood.
 α-Globulins and β-globulins, made by liver and other cells, include transferrin and other transport factors; fibronectin; prothrombin and other coagulation factors; lipoproteins and other proteins entering blood from tissues.
α-Globulins and β-globulins, made by liver and other cells, include transferrin and other transport factors; fibronectin; prothrombin and other coagulation factors; lipoproteins and other proteins entering blood from tissues.
 γ-Globulins, which are immunoglobulins (antibodies) secreted by plasma cells in many locations.
γ-Globulins, which are immunoglobulins (antibodies) secreted by plasma cells in many locations.
 Fibrinogen, the largest plasma protein (340 kD), also made in the liver, which, during clotting, polymerizes as insoluble, cross-linked fibers of fibrin that block blood loss from small vessels.
Fibrinogen, the largest plasma protein (340 kD), also made in the liver, which, during clotting, polymerizes as insoluble, cross-linked fibers of fibrin that block blood loss from small vessels.
 Complement proteins, a system of factors important in inflammation and destruction of microorganisms.
Complement proteins, a system of factors important in inflammation and destruction of microorganisms.
BLOOD CELLS
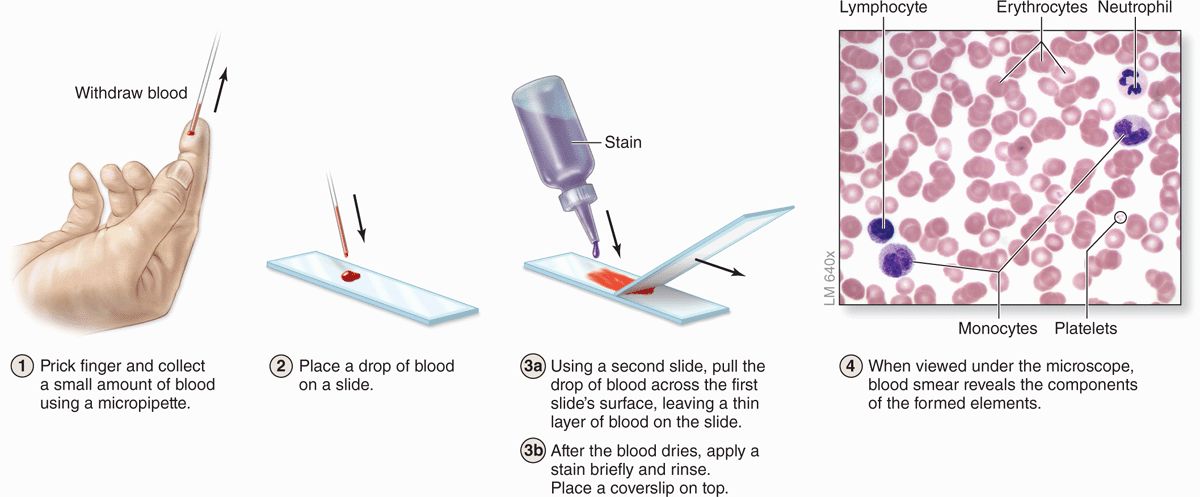
Blood cells can be studied histologically in smears prepared by spreading a drop of blood in a thin layer on a microscope slide (Figure 12–3). In such films the cells are clearly visible and distinct from one another, facilitating observation of their nuclei and cytoplasmic characteristics. Blood smears are routinely stained with special mixtures of acidic (eosin) and basic (methylene blue) dyes. These mixtures may also contain dyes called azures that are more useful in staining cytoplasmic granules containing charged proteins and proteoglycans. Azurophilic granules produce metachromasia in stained leukocytes like that seen with mast cells in connective tissue. Some of these special stains, such as Giemsa and Wright stain, are named after hematologists who introduced their own modifications into the original mixtures.
Erythrocytes
Erythrocytes (red blood cells or RBCs) are terminally differentiated structures lacking nuclei and completely filled with the O2-carrying protein hemoglobin. RBCs are the only blood cells whose function does not require them to leave the vasculature.
FIGURE 12–3 Preparing a blood smear.
Human erythrocytes suspended in an isotonic medium are flexible biconcave discs (Figure 12–4). They are approximately 7.5 μm in diameter, 2.6 μm thick at the rim, but only 0.75 μm thick in the center. Because of their uniform diameters and their presence in most tissue sections, RBCs can often be used by histologists as an internal standard to estimate the size of other cells or structures.
FIGURE 12–4 Normal human erythrocytes.
The biconcave shape provides a large surface-to-volume ratio and facilitates gas exchange. The normal concentration of erythrocytes in blood is approximately 3.9 to 5.5 million per microliter (μL, or mm3) in women and 4.1-6.0 million/μL in men.
Erythrocytes are normally quite flexible, which permits them to bend and adapt to the irregular turns and small diameters of capillaries. Observations in vivo show that at the angles of capillary bifurcations, erythrocytes with normal adult hemoglobin frequently assume a cuplike shape. In larger blood vessels RBCs often adhere to one another loosely in stacks called rouleaux (Figure 12–4c).
The plasmalemma of the erythrocyte, because of its ready availability, is the best-known membrane of any cell. It consists of about 40% lipid, 10% carbohydrate, and 50% protein. Most of the latter are integral membrane proteins (see Chapter 2), including ion channels, the anion transporter called band 3 protein, and glycophorin A. The glycosylated extracellular domains of the latter proteins include antigenic sites that form the basis for the ABO blood typing system. Several peripheral proteins are associated with the inner surface of the membrane, including spectrin, dimers of which form a lattice bound to underlying actin filaments, and ankyrin, which anchors the lattice to the glycophorins and band 3 proteins. This submembranous meshwork stabilizes the membrane, maintains the cell shape, and provides the cell elasticity required for passage through capillaries.
Erythrocyte cytoplasm lacks all organelles but is densely filled with hemoglobin, the tetrameric O2-carrying protein that accounts for the cells’ uniform acidophilia. When combined with O2 or CO2, hemoglobin forms oxyhemoglobin or carbaminohemoglobin, respectively. The reversibility of these combinations is the basis for the gas-transporting capability of hemoglobin. The combination of hemoglobin with carbon monoxide (CO) is irreversible, however, reducing the cells’ capacity to transport O2.
Erythrocyte differentiation (discussed in Chapter 13) includes loss of the nucleus and organelles, shortly before the cells are released by bone marrow into the circulation. Lacking mitochondria, erythrocytes rely on anaerobic glycolysis for their minimal energy needs. Lacking nuclei, they cannot replace defective proteins.
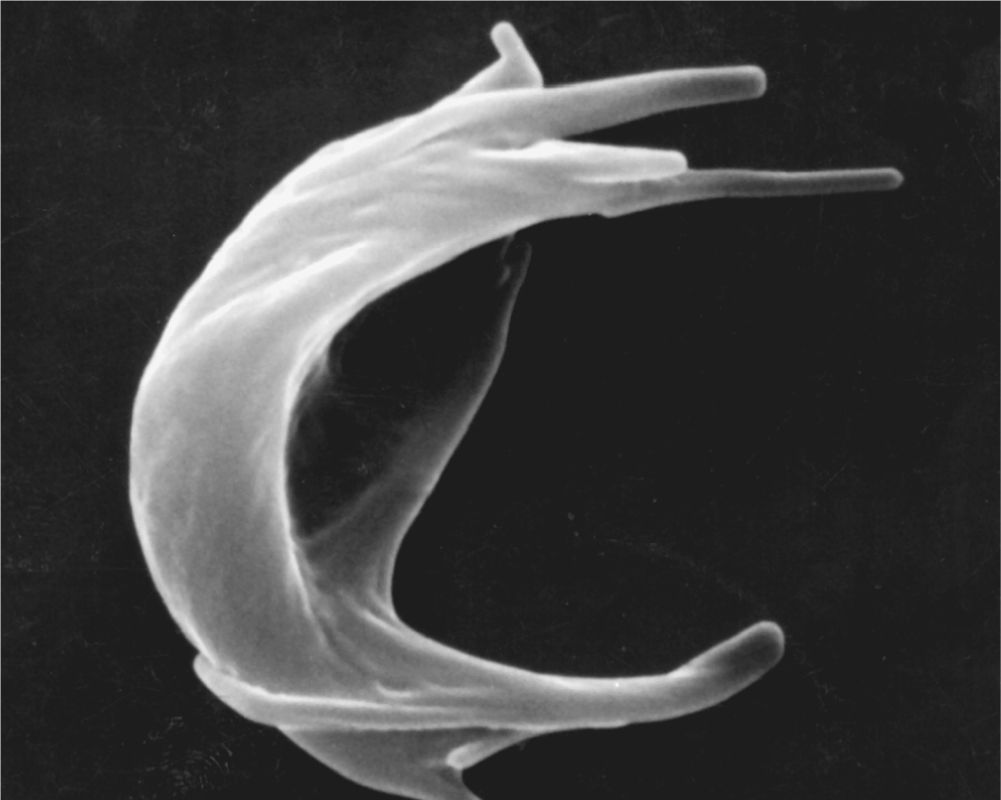
FIGURE 12–5 Sickle cell erythrocyte.
Human erythrocytes normally survive in the circulation for about 120 days. By this time defects in the membrane’s cytoskeletal lattice or ion transport systems begin to produce swelling or other shape abnormalities, as well as changes in the cells’ surface oligosaccharide complexes. Senescent or worn-out RBCs displaying such changes are removed from the circulation, mainly by macrophages of the spleen, liver, and bone marrow.
Leukocytes
Leukocytes (white blood cells or WBCs) leave the blood and migrate to the tissues where they become functional and perform various activities related to immunity. According to the type of cytoplasmic granules and their nuclear morphology, leukocytes are divided into two groups: granulocytes and agranulocytes (Table 12–2). Both types are rather spherical while suspended in blood plasma, but they become amoeboid and motile after leaving the blood vessels and invading the tissues. Their estimated sizes mentioned here refer to observations in blood smears in which the cells are spread and appear slightly larger than they are in the circulation.
TABLE 12–2 Leukocytes: Numbers, structural features, and major functions.
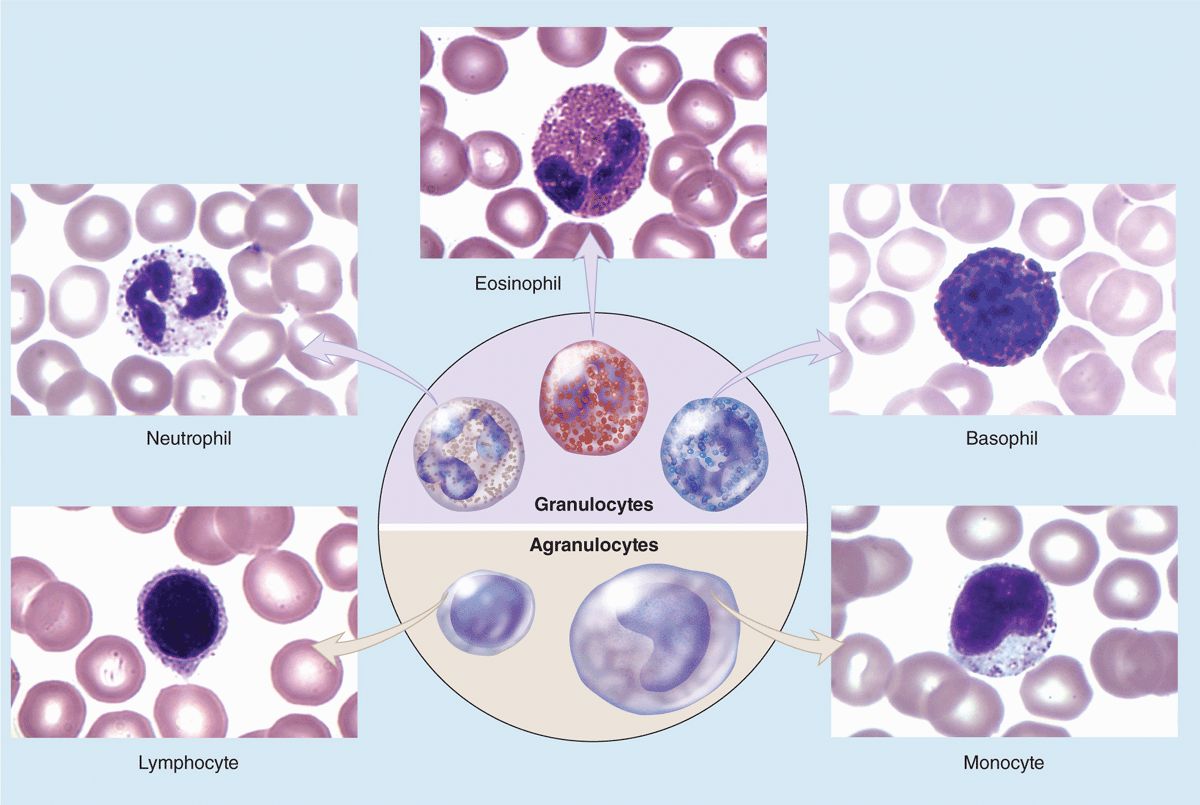
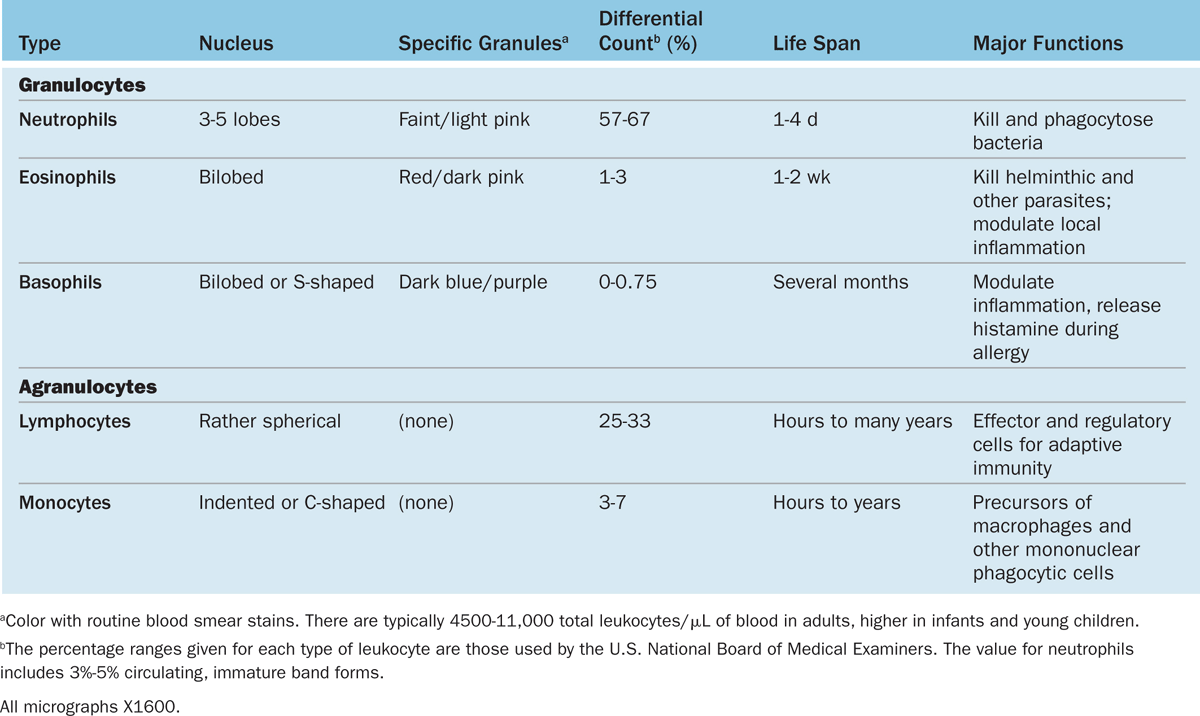
Granulocytes possess two major types of cytoplasmic granules: lysosomes (often called azurophilic granules in blood cells) and specific granules that bind neutral, basic, or acidic stains and have specific functions.
Granulocytes have polymorphic nuclei with two or more distinct (almost separated) nuclear lobes and include the neutrophils, eosinophils, and basophils (see Figure 12–1 and Table 12–2). All granulocytes are terminally differentiated cells with a life span of only a few days. Their Golgi complexes and rough ER are poorly developed. They have few mitochondria and depend largely on glycolysis for their low energy needs. Granulocytes normally die by apoptosis in the connective tissue and billions of neutrophils alone die by apoptosis each day in the adult human. The resulting cellular debris is removed by macrophages and, like all apoptotic cell death, does not itself elicit an inflammatory response.
Agranulocytes do not have specific granules, but they do contain azurophilic granules (lysosomes), with affinity for the basic stain azure A. The nucleus is spherical or indented but not lobulated. This group includes lymphocytes and monocytes (see Figure 12–1 and Table 12–2). The differential count (percentage of all leukocytes) for each type of leukocyte is also presented in Table 12–2.
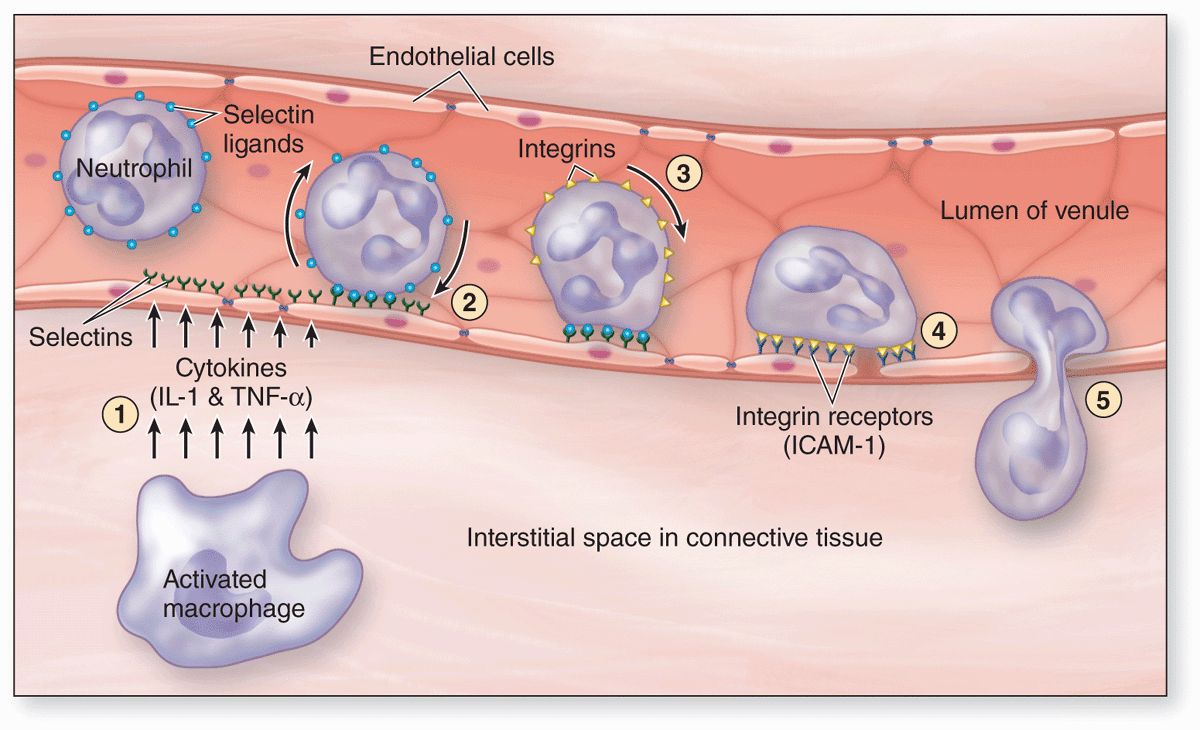
All leukocytes are key players in the defense against invading microorganisms, and in the repair of injured tissues, specifically leaving the microvasculature in injured or infected tissues. At such sites factors termed cytokines are released from various sources and these trigger loosening of intercellular junctions in the endothelial cells of local postcapillary venules (Figure 12–6). Simultaneously the cell adhesion protein P-selectin appears on these cells’ luminal surfaces produced by exocytosis of Weibel-Palade bodies. Neutrophils and other leukocytes have on their surfaces glycosylated ligands for P-selectin, and their interactions cause cells flowing through the venules to slow down, like rolling tennis balls arriving at a patch of velcro. Other cytokines stimulate the now slowly rolling leukocytes to express integrins and other adhesion factors that produce firm attachment to the endothelium (see Figure 11–21d). In a process called diapedesis (Gr. dia, through + pedesis, to leap), the leukocytes send extensions through the openings between the endothelial cells, migrate out of the venules into the surrounding tissue space, and head directly for the site of injury or invasion. The attraction of neutrophils to bacteria involves chemical mediators in a process of chemotaxis, which causes leukocytes to rapidly accumulate where their defensive actions are specifically needed.
FIGURE 12–6 Diagram of events involving leukocytes in a postcapillary venule at sites of inflammation.
 MEDICAL APPLICATION
MEDICAL APPLICATION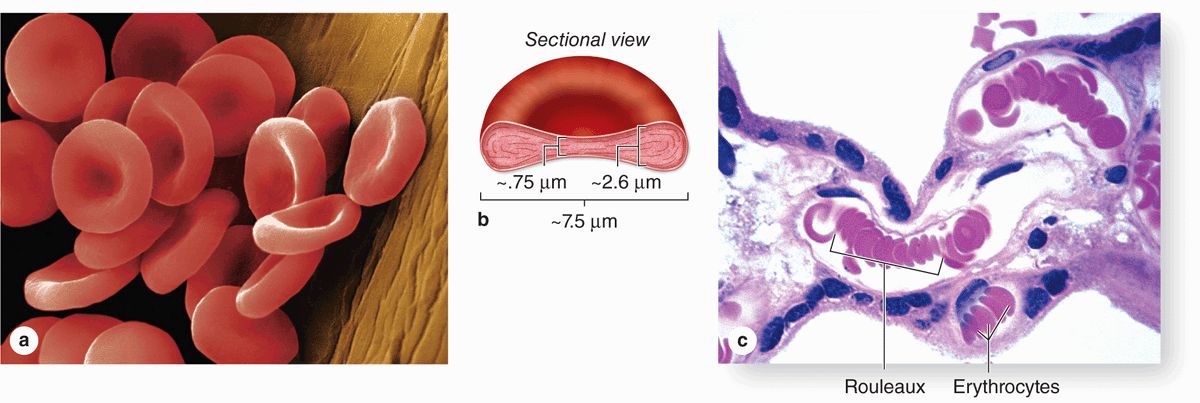
 MEDICAL APPLICATION
MEDICAL APPLICATION