Bleeding Duodenal Ulcer
Bruce David Schirmer
With each new addition of this text, the incidence of operative treatment of bleeding duodenal ulcer decreases. Nevertheless, the procedure is life saving and invaluable when needed for treatment of the life-threatening bleeding duodenal ulcer that is not amenable to lesser therapies due to temporal, situational, or clinical reasons. Thus, the procedure will be described within the context of other treatment options for this condition as well.
Medical therapy for duodenal ulcer has drastically decreased the need for surgery for this condition. Indications for surgery of any type for peptic ulcer disease now are limited to obstruction, bleeding, perforation, and, only in rare circumstances, symptoms refractoriness to medical therapy. Medical therapy has now effectively eliminated gastric acid production, with the initial class of histamine blocking drugs (H-2 blockers) leading the way 35 years ago, to be subsequently replaced largely now by the proton pump inhibitor (PPI) class of pharmacologic agents. PPIs eliminate the ability of the parietal cell to secrete hydrogen, effectively eliminating gastric acid secretion in most circumstances. The use of H-2 blockers alone resulted in the virtual elimination of elective gastric and surgery for peptic ulcer, while the PPIs have reinforced that trend. This process had already been accomplished when the bacterial causative agent for peptic ulcer, Helicobacter pylori, was discovered. Now the effective eradication of H. pylori by pharmacologic antibiotic and PPI combinations has lessened the incidence of peptic ulcer in the population even further.
Although these medications are well known and highly available to those patients in health care systems with the resources to supply them, there is still a significant portion of the world’s population who do not have access to such medications, and hence still may suffer the consequences of peptic ulcer disease. Similarly, noncomplicance with recommended therapy and use of ulcerogenic medications and practices that prevent ulcer healing (smoking is the primary one) still create the development of the peptic ulcer that requires treatment. Medical therapy is the first-line treatment for all noncomplicated peptic ulcer disease. It is the complicated peptic ulcer disease that still may require surgical therapy. Epidemiologic studies continue to demonstrate that the incidence of emergent and urgent interventional procedures for complications of peptic ulcer disease have not decreased to the same extent as the incidence of any elective surgical treatment for this condition.
The current indication for surgical therapy for treatment of bleeding duodenal ulcer is when
Less invasive therapies have failed
Less invasive therapies are not available
The severity of the bleeding does not allow for the potential for a lesser invasive procedure to be done in terms of the time allowable before the hemorrhage must be controlled (a hemodynamically unstable patient)
Determination of which therapeutic approach is indicated for each patient is a decision best reached by having the availability and mutual agreement of a multidiscipline team of gastroenterologists, surgeons, and radiologists, all of who can treat this problem and who agree on a protocol for the relative indications of each one’s therapeutic procedure.
Hematemesis is the most common manifestation of significant upper gastrointestinal bleeding. Melena may occur instead, or both may be present. Hemodynamic changes confirm the severity of the bleeding. A nasogastric tube is always indicated in patients with signs of sudden blood loss and no hematemesis, to help confirm or deny the presence of blood in the stomach. Massive duodenal bleeding usually has some duodenogastric reflux of blood even if no vomiting occurs. Thus, hematemesis or bloody nasogastric tube suctioning confirm upper gastrointestinal bleeding.
Knowledge of the patient’s medical history is important to focus the next steps on resuscitation and diagnosis prior to therapy. Patients with known cirrhosis and portal hypertension will be much more likely to have hematemesis on the basis of bleeding esophageal varices for example. For patients without any suggestion of liver disease or chronic alcohol ingestion, bleeding ulcer must be considered as the most likely cause for hemodynamically significant upper gastrointestinal hemorrhage.
Resuscitation
Without doubt, as a surgical consultant when I am asked to see a patient with upper gastrointestinal bleeding, the most common deficiency to that point in the overall treatment plan of the patient is usually a lack of adequate means for and accomplishment of resuscitation. As a long-standing colleague of mine once said, “There are more options with a live patient.” The patient with signs or suggestions of a significant upper gastrointestinal bleed should have the following resuscitative measures performed:
Large bore intravenous access, 16 gauge or larger, with two such lines available
Central venous access to monitor CVP and intravascular volume status
Foley catheter to monitor urine output
ICU bed with hemodynamic constant monitoring
Consideration of intubation if airway protection is indicated
Sufficient blood products available to meet the needs for resuscitation of both red cells and the capacity to clot normally
Such measures should be instituted before definitive diagnostic procedures are begun.
Diagnosis
Flexible upper endoscopy is the diagnostic procedure of choice. The surgeon is hopefully a skilled endoscopist and can proceed to perform the test. Otherwise, the gastroenterologist skilled in therapeutic endoscopy should have been consulted and be available to proceed. During the endoscopic procedure, continued monitoring of the patient’s vital signs and volume status is imperative, as is protection of the airway. The most frequent technical challenge to the endoscopist performing a successful diagnostic and potentially therapeutic intervention is the presence of a large amount of blood within the lumen of the stomach. Evacuation of this clot via a very large bore gastric lavage tube (32 French, Ewald tube) is mandatory. The tube is passed perorally and is capable of evacuating large clots. Lavage with several liters of water may be needed before adequate visualization may occur. The water should not be cold so as to avoid hypothermia from it.
Once the stomach lavage return is clearing, the flexible endoscope is introduced and the esophagus, stomach, and duodenum inspected to the end of the second portion of the duodenum. Virtually, all upper gastrointestinal bleeding sources are in this anatomic area. Bleeding lesions other than a duodenal ulcer may include esophagitis, esophageal varices, Mallory Weiss tear, Dieulafoy ulcer, gastritis, gastric varices, gastric ulcers, duodenitis, and tumors or polyps in any of these areas.
The typical bleeding duodenal ulcer is located several centimeters beyond the pylorus on the posterior aspect of the duodenum. The hemorrhage arises because the ulcer has eroded into a branch of or the main lumen of the gastroduodenal artery. If the latter has occurred, the bleeding is brisk and may be projectile.
Endoscopic Therapy
The endoscopist has several options for treating the bleeding duodenal ulcer. These include
Endoscopic clips placed directly on any visible vessel
Injection of the base of the ulcer with dilute epinephrine solution
Bicap probe to coapt the vessel lumen
Heater probe to coagulate the vessel lumen
Argon beam for more diffuse oozing surfaces (less likely for a bleeding vessel)
Skill and experience are needed to perform therapeutic endoscopy to arrest vigorous bleeding from a duodenal ulcer. The main technical problem is the ability to see through the scope despite the ongoing bleeding. The suction capacity of the endoscope is limited, and when used, performs the automatic negative process of removing the insufflated air within the lumen of the duodenum necessary to maintain visualization during the procedure.
Should the endoscopist fail, or should the patient rebleed after a visible vessel was seen, the patient is then best served by moving on to either radiologic or surgical therapy. If, however, the patient had hemostasis achieved with endoscopic means, and a second bleed of less severity occurs, and no large visible vessel was present in the ulcer, then usually another endoscopic procedure is indicated as the likelihood of repeat success is good.
Radiologic Therapy
Since the last edition of this text, the radiologic approach to upper gastrointestinal bleeding has become much more popular as the next therapeutic option should interventional endoscopy fail. Interventional radiologists in many institutions have developed increasing experience in vascular embolization to arrest hemorrhage over the past decade. The concern for end-organ ischemia that is present when embolization of organs such as the colon or small bowel is considered is not as great a concern with a bleeding duodenal artery, as the collateral circulation provided by the pancreaticoduodenal artery to the duodenum will usually prevent ischemia should the gastroduodenal be occluded from embolization. The radiologist uses a combination of coils or other intraluminal objects to achieve the desired embolization of the gastroduodenal artery. Success with this approach is good. However, in some circumstances, the backflow of the vessel or the volume of the bleeding is such that even this approach may fail. At that point, surgical therapy is the indicated course.
Radiologic intervention for bleeding duodenal ulcer is also ideal when the patient has failed endoscopic treatment and for a variety of reasons is a poor surgical candidate. These reasons could include inherent existing diseases of the patient, poor anesthetic risk, or multiple previous abdominal operations making abdominal access difficult, among others.
Although it is still controversial as to whether radiologic intervention is superior to surgical intervention in terms of overall outcomes for treating bleeding duodenal ulcer, it has now become the next step in many institution’s algorithms of treatment of bleeding duodenal ulcer after endoscopy and before surgery. The less invasive nature of the radiologic intervention has appeal in terms of limiting abdominal wall morbidity as well as other potential complications which may arise from abdominal surgery. This situation depends again on the local expertise available in any institution.
Surgical therapy must be exercised whenever the patient shows hemodynamic instability along the above algorithm, indicating that the ability to tolerate ongoing blood loss at that rate is limited in terms of time, and failure of an endoscopic or even radiologic approach could prove life threatening. This assumes that the patient is a candidate for surgical therapy. Primary emergency surgery is indicated when shock persists despite aggressive volume resuscitation and when blood loss continues, necessitating transfusion in excess of 6 units of blood over a 24-hour period. Other factors to consider in the decision-making process include patient older than 70 years (physiologically), the presence of multiple comorbidities, and a previous history of ulcer diathesis. Especially where interventional radiologic alternatives do not exist, the poorer the surgical candidate, often the more rapid and aggressive must be the surgical intervention if the patient is to survive. The timing of primary emergency surgery should be influenced by these factors, given that the morbidity and mortality are greatly increased with delayed intervention.
Choice of Operation
In choosing the type of operation for treatment of the actively bleeding duodenal ulcer, factors to be considered are the patient’s condition and the technical difficulty and expected duration of the operation. Local factors around the potential operative site including previous surgery and scarring must also be considered. The various operations which have been performed in the past have been well analyzed to allow some judgment as to their efficacy and appropriateness in the setting of treating duodenal ulcer.
While many operative procedures are now routinely done using a laparoscopic approach, the indications for surgical intervention for bleeding duodenal ulcer usually are such that one is performing an emergent operation where speed and simplicity are valuable components of the procedure. Laparoscopic opening of the duodenum and suturing of a bleeding ulcer may be quite within the technical abilities of some surgeons, and this approach, if able to be carried out with good visualization of the ulcer, is not to be ruled out. Nevertheless, the experience of most surgeons in rarely performing surgical therapy for bleeding duodenal ulcer and the technical difficulty of maintaining the pneumoperitoneum in the face of constant suctioning of blood from the bleeding vessel site make the laparoscopic approach limited in its application to a select few surgeons.
It is now also quite clear that the decades old recommendation of performing an antrectomy and truncal vagotomy as the most definitive treatment of peptic ulcer disease is also to be avoided in the setting of the emergent operation for bleeding duodenal ulcer. First, the duodenum must be opened to treat the ulcer, making the closure of the duodenum more difficult in the setting of antrectomy. The additional surgery needed for resection as well as anastomosis is not justified in the emergency setting.
We continue to advocate that the operation of choice for the high-risk, unstable patient continues to be suture ligation of the bleeding vessel, pyloroplasty, and truncal vagotomy. However, the availability of the
harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH) does allow the relatively rapid performance of a highly selective vagotomy, which avoids some of the potential side effects of the truncal vagotomy and may be safely applied should the patient be younger, healthier, and more hemodynamically stable. The anatomy of the nerves of Latarjet along the lesser curvature of the stomach must be intact and identifiable if the surgeon is to consider performing highly selective vagotomy in the emergency setting. Certainly, any vagotomy, truncal or highly selective, is the last step of the operation once hemostasis has been achieved and the duodenum is closed. At that point, the condition of the patient as well as other factors which may be relevant should combine to allow the surgeon to make the best decision as to proceeding with vagotomy and which type of vagotomy.
harmonic scalpel (Ethicon Endo-Surgery, Cincinnati, OH) does allow the relatively rapid performance of a highly selective vagotomy, which avoids some of the potential side effects of the truncal vagotomy and may be safely applied should the patient be younger, healthier, and more hemodynamically stable. The anatomy of the nerves of Latarjet along the lesser curvature of the stomach must be intact and identifiable if the surgeon is to consider performing highly selective vagotomy in the emergency setting. Certainly, any vagotomy, truncal or highly selective, is the last step of the operation once hemostasis has been achieved and the duodenum is closed. At that point, the condition of the patient as well as other factors which may be relevant should combine to allow the surgeon to make the best decision as to proceeding with vagotomy and which type of vagotomy.
Choice of Incision
An upper midline vertical incision serves most situations for performing upper abdominal surgery. The pylorus and duodenum are sufficiently close to the midline to allow good exposure with such an incision. The incision may need to be carried up to the xiphoid if truncal vagotomy is contemplated, especially if the patient is obese. The lower extent of the incision is usually around or above the umbilicus.
Upon entering the peritoneal cavity, rapid exploration should confirm the presence of a nasogastric tube or Ewald-type tube in the lumen of the stomach (at times from the endoscopy) and gastric decompression. The round and falciform ligaments are divided. Additional exposure of the duodenal area is achieved through the use of mechanical retractors. Several brands are available and afford adequate exposure. There should be an appropriate retractor for the liver to both retract it and avoid parenchymal injury. Human retractors are to be avoided in this setting for numerous reasons, not the least of which is reliability of exposure.
If a laparoscopic approach is used in a more elective setting, we use port placement similar to what we would use in the performance of an antireflux operation, but situating the surgeon’s right hand port closer toward the midline and the left port slightly further to the patient’s right. This allows the main axis of the camera, located in the umbilical region, to be pointed toward the duodenum. There is no place for single port surgery in this setting. A Nathanson, T-Boone, flexible triangular, or other epigastric to right subcostal located liver retractor is appropriate.
Suture Ligation
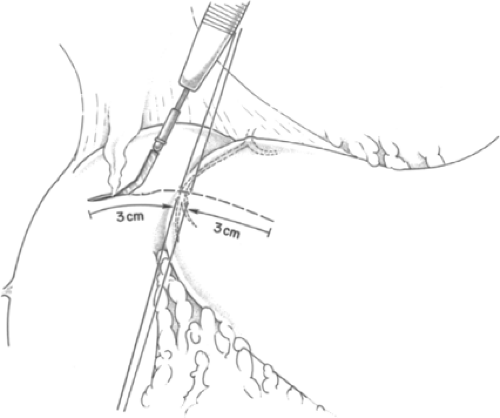
The primary objective during surgery to treat a bleeding duodenal ulcer is to control the bleeding vessel. Thus, this step takes precedence and is of paramount importance. When the bleeding vessel has been localized by endoscopy to be in the first portion of the duodenum, a Kocher maneuver is performed for mobilization of the second portion of the duodenum. The pyloric vein is used as a landmark to identify the pylorus, if it is not readily identifiable by visualization and palpation. Traction sutures of 2-0 silk are placed superior and inferior to the pyloric ring. A 3-cm-long incision is made centered on and through the pyloric ring, in the midportion of the anterior surface of the pylorus (Fig. 1). Traction on the sutures opens the incision into the form of a diamond, which allows adequate exposure. Intraluminal clot is suctioned away. Occasionally, the incision needs to be extended another 1 to 2 cm further into the duodenum if the ulcer is slightly more distal.
Once the pyloromyotomy is performed, the bleeding source and ulcer are identified. Direct pressure is applied to any ongoing bleeding while preparing to suture ligate the site. If the ulcer has a hematoma over it, the hematoma must be cleared away adequately to define the location of the bleeding vessel. If the bed of the ulcer has stopped actively bleeding, suture ligation is still performed. However, in this setting, a search for other potential bleeding sites should be performed. If it is clear that there is ongoing hemorrhage from a site more proximal to the pyloromyotomy from the stomach, attempts to intraoperatively visualize it through the incision should be made. Extension of the incision may be needed if the source is identified as close. If the source is unclear, intraoperative gastroscopy by a colleague is indicated to determine the site of the bleeding. Then a second gastrotomy can be made at that site to treat it appropriately.
When an actively bleeding duodenal ulcer is identified, direct pressure is followed by suture ligation. A thorough understanding of the anatomy of the gastroduodenal complex, described as the T-three vessel junction, is important when performing suture ligation (Fig. 2). The gastroduodenal complex should be transfixed at three points, with the gastroduodenal artery ligated proximal and distal to the site of penetration. The third suture ligature, a U stitch, should transfix the transverse pancreatic branch, which is located medially. Major rebleeding, if it occurs, is most likely due to imprecise placement of these suture ligatures through failure to recognize that the arterial site of bleeding is located at a junction of the gastroduodenal complex. We prefer to use heavy absorbable synthetic suture (2-0 weight), but the use of silk suture is acceptable. Heavier round surgical needles are indicated if there is excessively thick scarring of the posterior wall of the duodenum from
the ulcer, which is usually the case. Care must be taken to adequately incorporate the vessel into the ligature, but to avoid such a depth of penetration so as to avoid injury to any surrounding structures. The bile duct may be close enough to the suture that its avoidance must always be confirmed.
the ulcer, which is usually the case. Care must be taken to adequately incorporate the vessel into the ligature, but to avoid such a depth of penetration so as to avoid injury to any surrounding structures. The bile duct may be close enough to the suture that its avoidance must always be confirmed.
Pyloroplasty
The simplest closure of the pyloromyotomy is a standard Heineke-Mikulicz pyloroplasty. This involves simply closing the longitudinal opening in a transverse direction using a single layer of sutures. Running absorbable suture, usually of 3-0 weight, works adequately well. Prior to the laparoscopic era, much care was taken during closure of such openings in the gastrointestinal tract so as to invert mucosa and make the closure visually appealing. We now know from experience that closed is closed, and visible mucosal edges pose no risk for morbidity as long as the tissue has been adequately closed together. Simple running suture suffices. More technically involved suture techniques such as the Gambee suture are proven traditional methods as well. This suture technique involves a full thickness penetration of the bowel from serosa to mucosa on one side, then a submucosal bite on that same side intraluminally, followed by a mirror image submucosal bite in the bowel inner surface on the opposite side of the opening, then a full thickness from mucosa out through serosa of the second bowel wall. This is illustrated in Figure 3, which shows closing in a Heineke-Mikulicz fashion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree