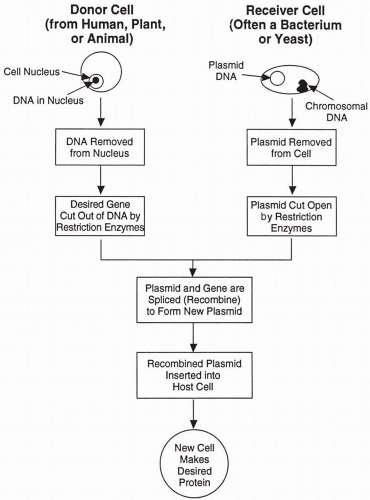
The design and creation of specific deoxyribonucleic acid (
DNA) sequences, as well as gene splicing with selected pieces of naturally-occurring
DNA, are used along with established and productive cell lines to obtain a unique cell that manufactures the desired product. A portion of the genetic material (i.e.,
DNA) that includes a gene from one species (usually mammalian and often human) is removed from the chromosome or reverse transcribed from messenger ribonucleic acic (RNA) (complementary
DNA) and spliced into the
DNA of a second species. The
DNA pieces are thus recombined (thus explaining the term
recombinant DNA) and information in the
DNA from the first species is transcribed and translated into protein using the production capability of the second. Thus, if the gene from the first species (e.g., human) coded for a specific protein that could be used as a drug (e.g., tissue plasminogen activator, insulin, or human growth hormone), then the bacteria, yeast or mammalian cell line that received this human gene is able to produce the desired human protein. As the cell with recombined
DNA continues to divide and re-divide, all of its progeny contain the genetic ability to manufacture the same human protein, although the stability of the foreign-
DNA insert can vary and must be meticulously monitored. In order to produce the protein needed, a company enables many cell divisions to occur from working cell banks, derived from the master cell bank, and using various scales of fermentation from a few hundred liters to many thousands as appropriate to the amounts and nature of the protein needed. After the original cell has divided into billions of cells (amplification) and production of the desired protein is induced, the protein product is harvested by separation from the cells. The product then undergoes a series of purification steps. Biologics
produced in this way include alpha interferon, tissue plasminogen activator, erythropoietin, and human growth hormone. For the early biotech products the second species was usually a bacterium [e.g.,
Escherichia coli (E. coli)], and later yeast cell lines were develop to act as the factory to make the protein of interest. The choice of cell line is critical to the nature of the product. For example, bacterial cell lines such as
E. coli cannot make glycosylated proteins (those with additional sugars attached) whereas yeasts can. The more recently developed mammalian cell lines, such as
CHO (derived from Chinese hamster ovary cells), can be more difficult to culture and grow, but have further attributes critical to the post-translational aspects of the recombinant product that may be critical to its ultimate ability to function in the human body in the way needed for therapeutic effect.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access
