23
Biosynthesis of Fatty Acids & Eicosanoids
Kathleen M. Botham, PhD, DSc & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() Describe the reaction catalyzed by acetyl-CoA carboxylase and understand the mechanisms by which its activity is regulated to control the rate of fatty acid synthesis.
Describe the reaction catalyzed by acetyl-CoA carboxylase and understand the mechanisms by which its activity is regulated to control the rate of fatty acid synthesis.
![]() Outline the structure of the fatty acid synthase multienzyme complex, indicating the sequence of enzymes in the two peptide chains of the homodimer.
Outline the structure of the fatty acid synthase multienzyme complex, indicating the sequence of enzymes in the two peptide chains of the homodimer.
![]() Explain how long-chain fatty acids are synthesized by the repeated condensation of two carbon units, with formation of the 16-carbon palmitate being favored in most tissues, and identify the co-factors required.
Explain how long-chain fatty acids are synthesized by the repeated condensation of two carbon units, with formation of the 16-carbon palmitate being favored in most tissues, and identify the co-factors required.
![]() Indicate the sources of reducing equivalents (NADPH) for fatty acid synthesis.
Indicate the sources of reducing equivalents (NADPH) for fatty acid synthesis.
![]() Understand how fatty acid synthesis is regulated by nutritional status and identify other control mechanisms that operate in addition to modulation of the activity of acetyl-CoA carboxylase.
Understand how fatty acid synthesis is regulated by nutritional status and identify other control mechanisms that operate in addition to modulation of the activity of acetyl-CoA carboxylase.
![]() Identify the nutritionally essential fatty acids and explain why they cannot be formed in the body.
Identify the nutritionally essential fatty acids and explain why they cannot be formed in the body.
![]() Explain how polyunsaturated fatty acids are synthesized by desaturase and elongation enzymes.
Explain how polyunsaturated fatty acids are synthesized by desaturase and elongation enzymes.
![]() Outline the cyclooxygenase and lipoxygenase pathways responsible for the formation of the various classes of eicosanoids.
Outline the cyclooxygenase and lipoxygenase pathways responsible for the formation of the various classes of eicosanoids.
BIOMEDICAL IMPORTANCE
Fatty acids are synthesized by an extramitochondrial system, which is responsible for the complete synthesis of palmitate from acetyl-CoA in the cytosol. In most mammals, glucose is the primary substrate for lipogenesis, but in ruminants it is acetate, the main fuel molecule produced by the diet. Critical diseases of the pathway have not been reported in humans. However, inhibition of lipogenesis occurs in type 1 (insulin-dependent) diabetes mellitus, and variations in the activity of the process affect the nature and extent of obesity.
Unsaturated fatty acids in phospholipids of the cell membrane are important in maintaining membrane fluidity. A high ratio of polyunsaturated fatty acids to saturated fatty acids (P:S ratio) in the diet is considered to be beneficial in preventing coronary heart disease. Animal tissues have limited capacity for desaturating fatty acids, and require certain dietary polyunsaturated fatty acids derived from plants. These essential fatty acids are used to form eicosanoic (C20) fatty acids, which give rise to the eicosanoids prostaglandins, thromboxanes, leukotrienes, and lipoxins. Prostaglandins mediate inflammation, pain, and induce sleep and also regulate blood coagulation and reproduction. Nonsteroidal anti-inflammatory drugs (NSAIDs) such as aspirin and ibuprofen act by inhibiting prostaglandin synthesis. Leukotrienes have muscle contractant and chemotactic properties and are important in allergic reactions and inflammation.
THE MAIN PATHWAY FOR DE NOVO SYNTHESIS OF FATTY ACIDS (LIPOGENESIS) OCCURS IN THE CYTOSOL
This system is present in many tissues, including liver, kidney, brain, lung, mammary gland, and adipose tissue. Its cofactor requirements include NADPH, ATP, Mn2+, biotin, and HCO3– (as a source of CO2). Acetyl-CoA is the immediate substrate, and free palmitate is the end product.
Production of Malonyl-CoA Is the Initial & Controlling Step in Fatty Acid Synthesis
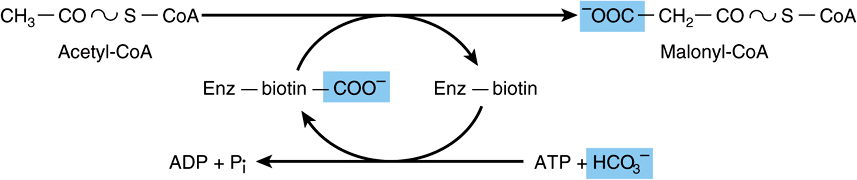
Bicarbonate as a source of CO2 is required in the initial reaction for the carboxylation of acetyl-CoA to malonyl-CoA in the presence of ATP and acetyl-CoA carboxylase. Acetyl-CoA carboxylase has a requirement for the B vitamin biotin (Figure 23–1). The enzyme is a multienzyme protein containing a variable number of identical subunits, each containing biotin, biotin carboxylase, biotin carboxyl carrier protein, and transcarboxylase, as well as a regulatory allosteric site. The reaction takes place in two steps: (1) carboxylation of biotin involving ATP and (2) transfer of the carboxyl group to acetyl-CoA to form malonyl-CoA.
FIGURE 23–1 Biosynthesis of malonyl-CoA. (Enz, acetyl-CoA carboxylase.)
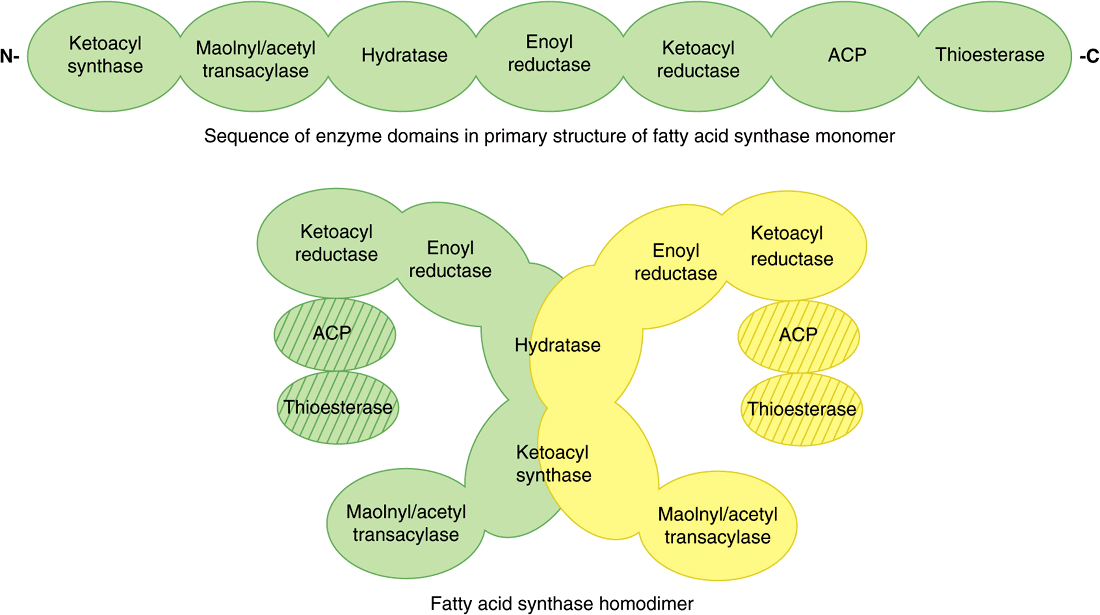
The Fatty Acid Synthase Complex Is a Homodimer of Two Polypeptide Chains Containing Six Enzyme Activities
In mammals, the individual enzymes of the fatty acid synthase system are linked in a multienzyme polypeptide complex that incorporates the acyl carrier protein (ACP), which takes over the role of CoA. It contains the vitamin pantothenic acid in the form of 4′-phosphopantetheine (Figure 44–18). In the primary structure of the protein, the enzyme domains are linked in the sequence as shown in Figure 23–2. X-ray crystallography of the three-dimensional structure, however, has shown that the complex is a homodimer, with two identical subunits, each containing 6 enzymes and an ACP, arranged in an X shape (Figure 23–2). The position of the ACP and thioesterase domains cannot be resolved as yet by X-ray crystallography, possibly because they are too flexible, but they are thought to lie close to the 3-ketoacylreductase enzyme. The use of one multienzyme functional unit has the advantages of achieving the effect of compartmentalization of the process within the cell without the erection of permeability barriers, and synthesis of all enzymes in the complex is coordinated since it is encoded by a single gene.
FIGURE 23–2 Fattyacid synthase multienzymecomplex. The complex is a dimeroftwo identical polypeptide monomers in which six enzymes and the acyl carrier protein (ACP) are linked in the primary structure in the sequence shown. X-ray crystallography of the three-dimensional structure has demonstrated that the two monomers in the complex are arranged in an X-shape. The position of the ACP and thioesterase is not yet resolved, but they are thought to be close to the 3 ketoacylreductase enzyme domain.
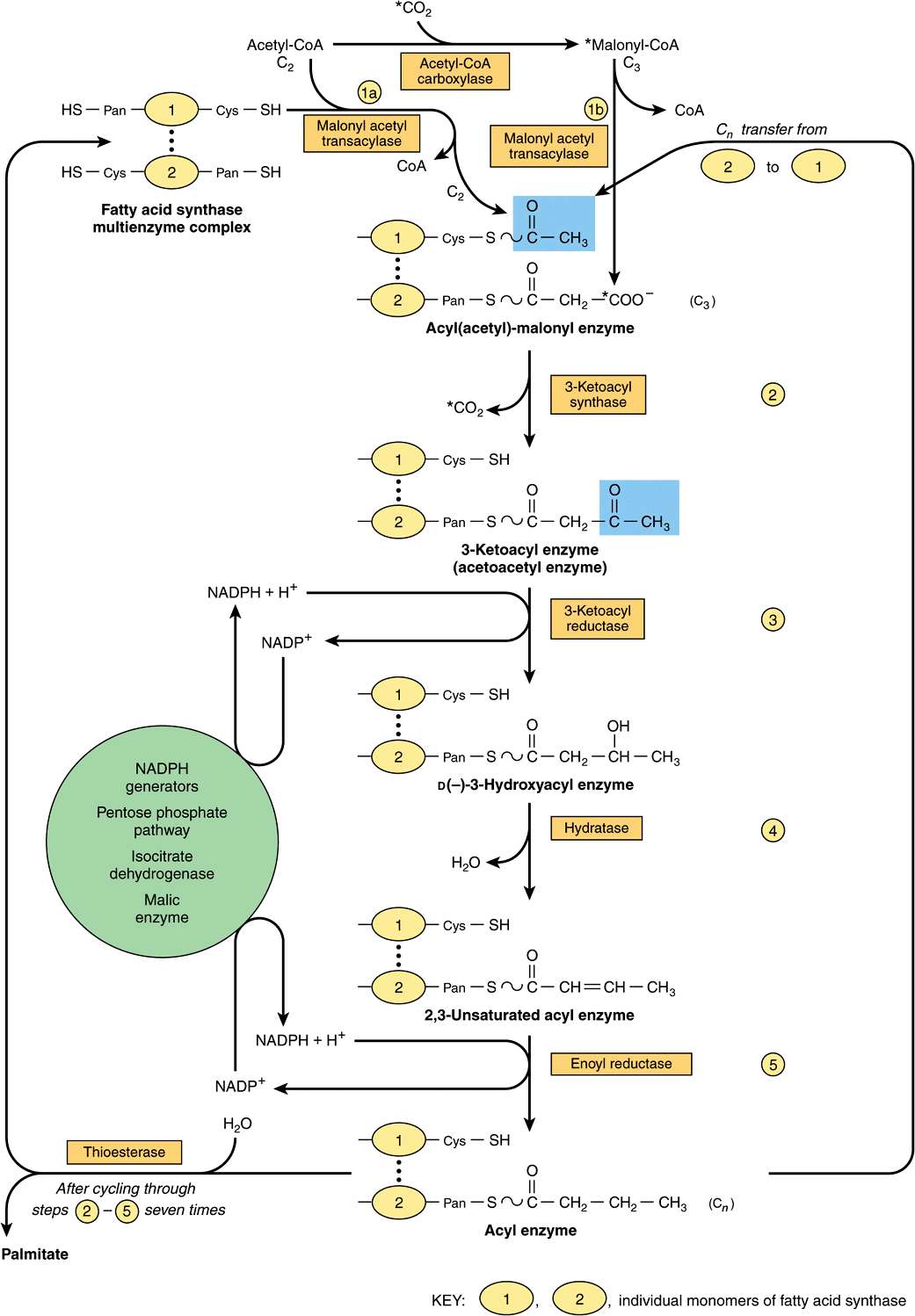
Initially, a priming molecule of acetyl-CoA combines with a cysteine—SH group (Figure 23–3, reaction 1a), while malonyl-CoA combines with the adjacent—SH on the 4′-phosphopantetheine of ACP of the other monomer (reaction 1b). These reactions are catalyzed by malonyl acetyl transacylase, to form acetyl (acyl)-malonyl enzyme. The acetyl group attacks the methylene group of the malonyl residue, catalyzed by 3-ketoacyl synthase, and liberates CO2, forming 3-ketoacyl enzyme (acetoacetyl enzyme) (reaction 2), freeing the cysteine—SH group. Decarboxylation allows the reaction to go to completion, pulling the whole sequence of reactions in the forward direction. The 3-ketoacyl group is reduced, dehydrated, and reduced again (reactions 3-5) to form the corresponding saturated acyl-S-enzyme. A new malonyl-CoA molecule combines with the—SH of 4′-phosphopantetheine, displacing the saturated acyl residue onto the free cysteine—SH group. The sequence of reactions is repeated six more times until a saturated 16-carbon acyl radical (palmitoyl) has been assembled. It is liberated from the enzyme complex by the activity of the sixth enzyme in the complex, thioesterase (deacylase). The free palmitate must be activated to acyl-CoA before it can proceed via any other metabolic pathway. Its possible fates are esterification into acylglycerols, chain elongation or de-saturation, or esterification to cholesteryl ester. In mammary gland, there is a separate thioesterase specific for acyl residues of C8, C10, or C12, which are subsequently found in milk lipids.
FIGURE 23–3 Biosynthesis oflong-chain fattyacids. Details ofhowaddition ofa malonyl residue causes the acyl chain to grow by two carbon atoms. (Cys, cysteine residue; Pan, 4′-phosphopantetheine.) The blocks highlighted in blue contain initially a C2 unit derived from acetyl-CoA (as illustrated) and subsequently the Cn unit formed in reaction 5.
The equation for the overall synthesis of palmitate from acetyl-CoA and malonyl-CoA is
![]()
The acetyl-CoA used as a primer forms carbon atoms 15 and 16 of palmitate. The addition of all the subsequent C. units is via malonyl-CoA. Propionyl CoA acts as primer for the synthesis of long-chain fatty acids having an odd number of carbon atoms, found particularly in ruminant fat and milk.
The Main Source of NADPH for Lipogenesis Is the Pentose Phosphate Pathway
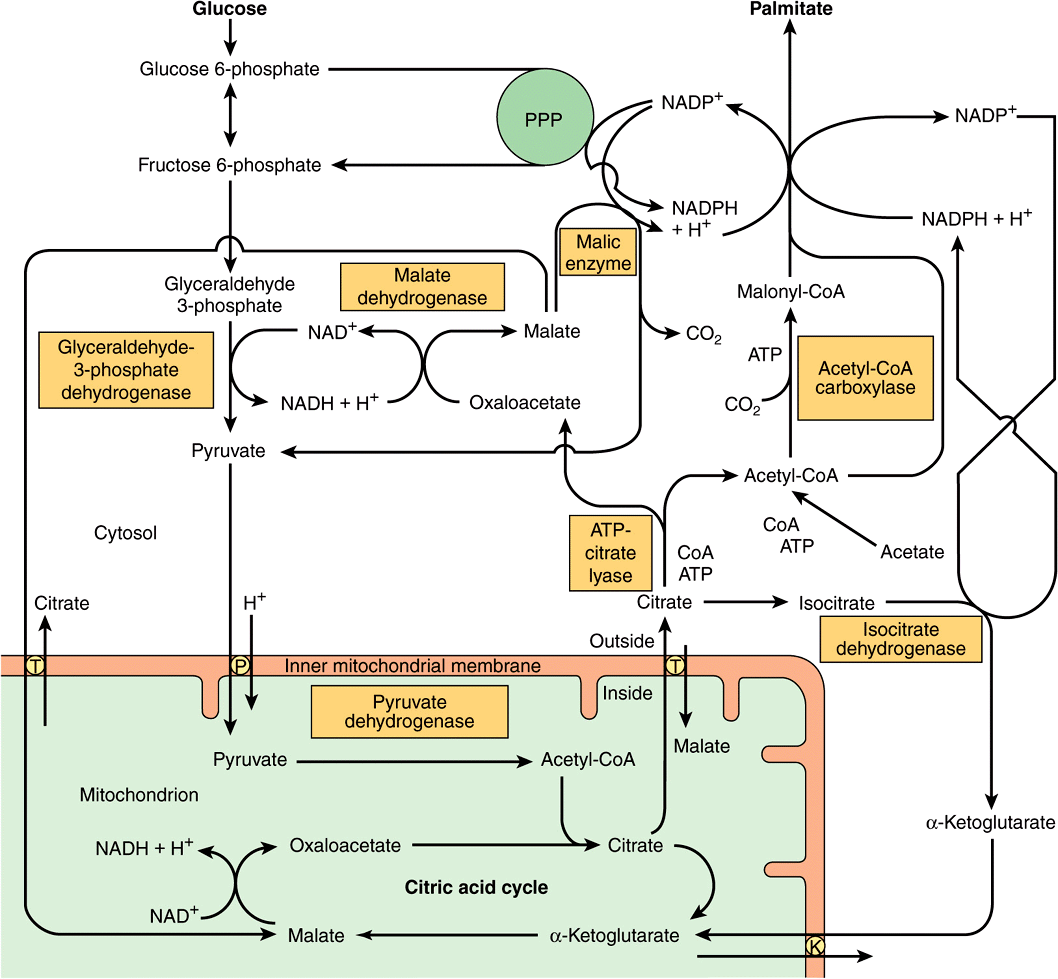
NADPH is involved as donor of reducing equivalents in both the reduction of the 3-ketoacyl and of the 2,3-unsaturated acyl derivatives (Figure 23–3. reactions 3 and 5). The oxidative reactions of the pentose phosphate pathway (see Chapter 21) are the chief source of the hydrogen required for the reductive synthesis of fatty acids. Significantly, tissues specializing in active lipogenesis—ie, liver, adipose tissue, and the lactating mammary gland—also possess an active pentose phosphate pathway. Moreover, both metabolic pathways are found in the cytosol of the cell; so, there are no membranes or permeability barriers against the transfer of NADPH. Other sources of NADPH include the reaction that converts malate to pyruvate catalyzed by the “malic enzyme” (NADP malate dehydrogenase) (Figure 23–4) and the extramitochondrial isocitrate dehydrogenase reaction (probably not a substantial source, except in ruminants).
FIGURE 23–4 The provision of acetyl-CoA and NADPH for lipogenesis. (K, α-ketoglutarate transporter; P, pyruvate transporter; PPP, pentose phosphate pathway; T, tricarboxylate transporter.)
Acetyl-CoA Is the Principal Building Block of Fatty Acids
Acetyl-CoA is formed from glucose via the oxidation of pyruvate within the mitochondria. However, it does not diffuse readily into the extramitochondrial cytosol, the principal site of fatty acid synthesis. Citrate, formed after condensation of acetyl-CoA with oxaloacetate in the citric acid cycle within mitochondria, is translocated into the extramitochondrial compartment via the tricarboxylate transporter, where in the presence of CoA and ATP, it undergoes cleavage to acetyl-CoA and oxaloacetate catalyzed by ATP-citrate lyase, which increases in activity in the well-fed state. The acetyl-CoA is then available for malonyl-CoA formation and synthesis to palmitate (Figure 23–4). The resulting oxaloacetate can form malate via NADH-linked malate dehydrogenase, followed by the generation of NADPH via the malic enzyme. The NADPH becomes available for lipogenesis, and the pyruvate can be used to regenerate acetyl-CoA after transport into the mitochondrion. This pathway is a means of transferring reducing equivalents from extramitochondrial NADH to NADP. Alternatively, malate itself can be transported into the mitochondrion, where it is able to re-form oxaloacetate. Note that the citrate (tricarboxylate) transporter in the mitochondrial membrane requires malate to exchange with citrate (see Figure 13–10). There is little ATP-citrate lyase or malic enzyme in ruminants, probably because in these species acetate (derived from carbohydrate digestion in the rumen and activated to acetyl-CoA extramitochondrially) is the main source of acetyl-CoA.
Elongation of Fatty Acid Chains Occurs in the Endoplasmic Reticulum
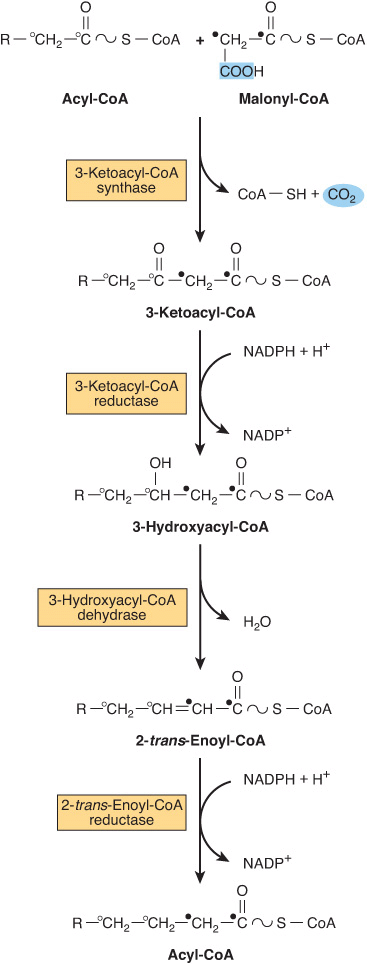
This pathway (the “microsomal system”) elongates saturated and unsaturated fatty acyl-CoAs (from C10 upward) by two carbons, using malonyl-CoA as the acetyl donor and NADPH as the reductant, and is catalyzed by the microsomal fatty acid elongase system of enzymes (Figure 23–5). Elongation of stearyl-CoA in brain increases rapidly during myelination in order to provide C22 and C24 fatty acids for sphingolipids.
FIGURE 23–5 Microsomal elongase system for fatty acid chain elongation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree