11
Bioenergetics: The Role of ATP
Kathleen M. Botham, PhD, DSc & Peter A. Mayes, PhD, DSc
OBJECTIVES
After studying this chapter, you should be able to:
![]() State the first and second laws of thermodynamics and understand how they apply to biologic systems.
State the first and second laws of thermodynamics and understand how they apply to biologic systems.
![]() Explain what is meant by the terms free energy, entropy, enthalpy, exergonic, and endergonic.
Explain what is meant by the terms free energy, entropy, enthalpy, exergonic, and endergonic.
![]() Appreciate how reactions that are endergonic may be driven by coupling to those that are exergonic in biologic systems.
Appreciate how reactions that are endergonic may be driven by coupling to those that are exergonic in biologic systems.
![]() Understand the role of high-energy phosphates, ATP, and other nucleotide triphosphates in the transfer of free energy from exergonic to endergonic processes, enabling them to act as the “energy currency” of cells.
Understand the role of high-energy phosphates, ATP, and other nucleotide triphosphates in the transfer of free energy from exergonic to endergonic processes, enabling them to act as the “energy currency” of cells.
BIOMEDICAL IMPORTANCE
Bioenergetics, or biochemical thermodynamics, is the study of the energy changes accompanying biochemical reactions. Biologic systems are essentially isothermic and use chemical energy to power living processes. How an animal obtains suitable fuel from its food to provide this energy is basic to the understanding of normal nutrition and metabolism. Death from starvation occurs when available energy reserves are depleted, and certain forms of malnutrition are associated with energy imbalance (marasmus). Thyroid hormones control the rate of energy release (metabolic rate), and disease results when they malfunction. Excess storage of surplus energy causes obesity, an increasingly common disease of Western society, which predisposes to many diseases, including cardiovascular disease and diabetes mellitus type 2, and lowers life expectancy.
FREE ENERGY IS THE USEFUL ENERGY IN A SYSTEM
Gibbs change in free energy (AG) is that portion of the total energy change in a system that is available for doing work—ie, the useful energy, also known as the chemical potential.
Biologic Systems Conform to the General Laws of Thermodynamics
The first law of thermodynamics states that the total energy of a system, including its surroundings, remains constant. It implies that within the total system, energy is neither lost nor gained during any change. However, energy may be transferred from one part of the system to another or may be transformed into another form of energy. In living systems, chemical energy may be transformed into heat or into electrical, radiant, or mechanical energy.
The second law of thermodynamics states that the total entropy of a system must increase if a process is to occur spontaneously. Entropy is the extent of disorder or randomness of the system and becomes maximum as equilibrium is approached. Under conditions of constant temperature and pressure, the relationship between the free-energy change (ΔG) of a reacting system and the change in entropy (ΔS) is expressed by the following equation, which combines the two laws of thermodynamics:
![]()
where ΔH is the change in enthalpy (heat) and T is the absolute temperature.
In biochemical reactions, since ΔH is approximately equal to ΔE, the total change in internal energy of the reaction, the above relationship may be expressed in the following way:
![]()
If ΔG is negative, the reaction proceeds spontaneously with loss of free energy; ie, it is exergonic. If, in addition, ΔG is of great magnitude, the reaction goes virtually to completion and is essentially irreversible. On the other hand, if ΔG is positive, the reaction proceeds only if free energy can be gained; ie, it is endergonic. If, in addition, the magnitude of ΔG is great, the system is stable, with little or no tendency for a reaction to occur. If ΔG is zero, the system is at equilibrium and no net change takes place.
When the reactants are present in concentrations of 1.0 mol/L, ΔG0 is the standard free-energy change. For biochemical reactions, a standard state is defined as having a pH of 7.0. The standard free-energy change at this standard state is denoted by ΔG0’.
The standard free-energy change can be calculated from the equilibrium constant Keq.
![]()
where R is the gas constant and T is the absolute temperature (Chapter 8). It is important to note that the actual ΔG may be larger or smaller than ΔG0’ depending on the concentrations of the various reactants, including the solvent, various ions, and proteins.
In a biochemical system, an enzyme only speeds up the attainment of equilibrium; it never alters the final concentrations of the reactants at equilibrium.
ENDERGONIC PROCESSES PROCEED BY COUPLING TO EXERGONIC PROCESSES
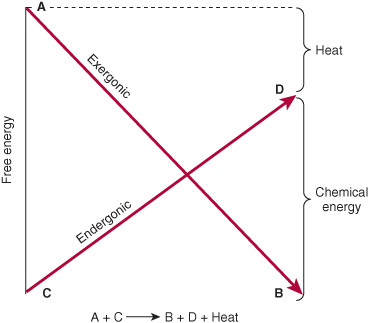
The vital processes—eg, synthetic reactions, muscular contraction, nerve impulse conduction, and active transport—obtain energy by chemical linkage, or coupling, to oxidative reactions. In its simplest form, this type of coupling may be represented as shown in Figure 11–1. The conversion of metabolite A to metabolite B occurs with release of free energy and is coupled to another reaction in which free energy is required to convert metabolite C to metabolite D. The terms exergonic and endergonic, rather than the normal chemical terms “exothermic” and “endothermic,” are used to indicate that a process is accompanied by loss or gain, respectively, of free energy in any form, not necessarily as heat. In practice, an endergonic process cannot exist independently, but must be a component of a coupled exergonic-endergonic system where the overall net change is exergonic. The exergonic reactions are termed catabolism (generally, the breakdown or oxidation of fuel molecules), whereas the synthetic reactions that build up substances are termed anabolism. The combined catabolic and anabolic processes constitute metabolism.
FIGURE 11–1 Coupling of an exergonic to an endergonic reaction.
If the reaction shown in Figure 11–1 is to go from left to right, then the overall process must be accompanied by loss of free energy as heat. One possible mechanism of coupling could be envisaged if a common obligatory intermediate (I) took part in both reactions, ie,
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree