Benign Papillary Tumors
INTRADUCTAL PAPILLOMA
A papilloma is a discrete, benign papillary tumor that arises most often in the central part of the breast from a lactiferous duct, but it can occur in any quadrant. An intracystic papilloma is a papilloma in a cystically dilated duct. Solid, noncystic papillomas have been classified as ductal adenomas or adenomyoepitheliomas. Papillomas may be solitary, consisting of a single papillary tumor in one duct, or multiple usually growing in contiguous branches of the ductal system. Intraductal papillomas should be distinguished from papillomatosis (epitheliosis), a term that describes microscopic duct hyperplasia. Papillomatosis may, and often does, coexist with solitary or multiple papillomas.
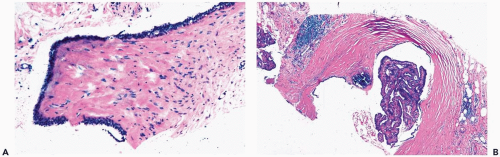
A subareolar mass may be palpable in a patient with a central solitary papilloma, and a palpable tumor can be the first clinical manifestation of a papilloma in one of the quadrants. Cystic papillomas appear to be well circumscribed on mammography (1) (Fig. 4.1). The presence of a cystic component is best appreciated by ultrasonography. Solitary papillomas occur at any age from infancy to the ninth decade. Nipple discharge occurs in most patients with a central papilloma. Bloody discharge, more commonly associated with papillary carcinoma, can be caused by degenerative changes in a papilloma.
Multiple papillomas develop more often peripherally than centrally and typically cause a palpable lesion. These patients tend to be younger on average than women with solitary papillomas, often presenting in their 40s and early 50s.
Part of the mass associated with a solitary papilloma may be the cyst formed by a dilated duct in which the papilloma arose. Some papillomas obliterate the cystic space. These solid papillary lesions are well circumscribed and appear to be enclosed in a capsule formed by the duct wall and accompanying reactive changes. Solitary papillomas can occur as clinically symptomatic tumors, 1 cm or less in diameter, in a major lactiferous duct. Small, asymptomatic peripheral papillomas may be detected by mammography. The average size of palpable lesions is 2 to 3 cm. Cystic papillomas can be larger than 10 cm.
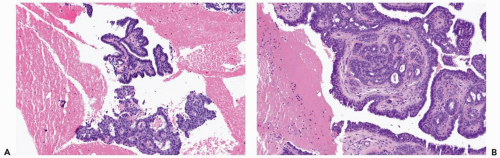
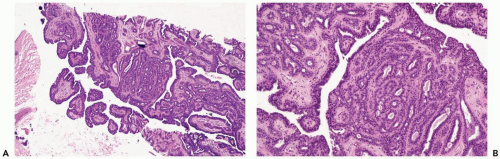
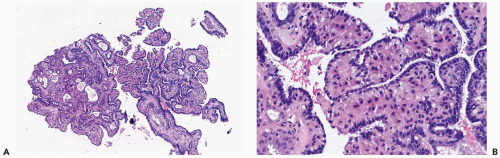
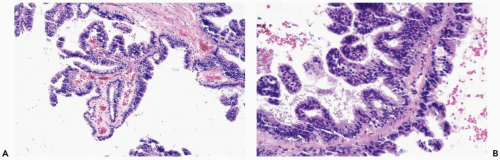
The most orderly form of papilloma consists of branching fronds of stroma supporting a layer of epithelium composed of cuboidal to columnar epithelial cells and myoepithelial cells (Fig. 4.2). The papillary stroma may arise from a single base or from several sites in the duct wall. The nonpapillary portion of the duct usually exhibits little or no epithelial hyperplasia in such lesions (Fig. 4.3).
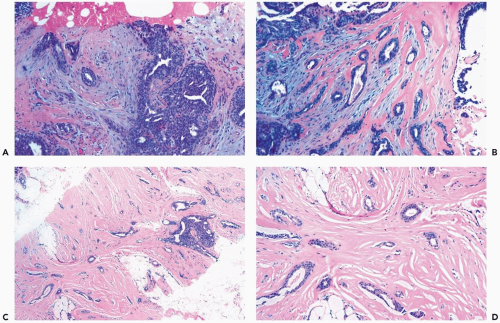
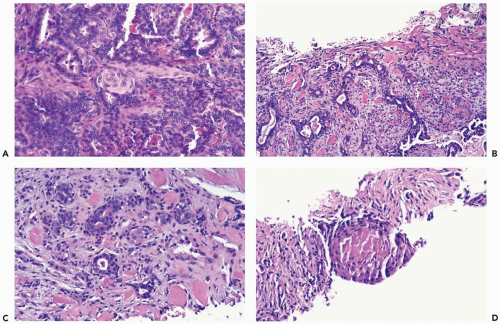
Some papillomas have a more complex structure caused by stromal overgrowth, hyperplasia of the epithelium, or both processes resulting in fusion of papillary fronds. Secondary microlumens are formed within the hyperplastic epithelium, and there may be micropapillary epithelial hyperplasia (Fig. 4.4). Proliferation of epithelium in the midst of the fibrovascular stroma results in a pattern resembling sclerosing adenosis (Figs. 4.5, 4.6, 4.7). The most florid form of epithelial hyperplasia is the solid intraductal papilloma, in which virtually all space between fibrovascular stalks is filled by proliferative duct epithelium (Fig. 4.8). Cytologically bland or mildly atypical apocrine epithelium is almost always an indication that a papillary tumor is a papilloma (Fig. 4.9). Rarely, there may be focal intraductal carcinoma in such a papilloma. Apocrine atypia manifested by nuclear pleomorphism and cytoplasmic clearing is more likely to be encountered in sclerosing papillary tumors (2).
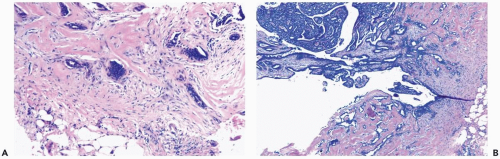
In many papillomas, the stroma is limited to a network of slender inconspicuous bands consisting of thin-walled capillaries accompanied by sparse fibroblasts, collagen, and mononuclear cells. Collagenization of the fibrovascular stroma occurs in some papillomas. The presence of histocytes in the stroma of papillary fronds almost always indicates that the lesion is a papilloma (Fig. 4.3 and 4.9A). The papillary architecture is accentuated when sclerosis is limited to the intrinsic papillary structure. If myofibroblastic proliferation accompanies collagenization of the stroma, the papillary arrangement is likely to become distorted (Fig. 4.10). Epithelial elements entrapped in this stroma may simulate invasive carcinoma within the lesion or at the periphery
(Fig. 4.11). When immunostains are done to detect myoepithelial cells, it is necessary to distinguish between stromal and myoepithelial reactivity. Fibrous sclerosis can be so severe that it virtually obliterates the papilloma, reducing it to a nodular scar containing scattered, benign glandular elements that may be difficult to distinguish from a fibroadenoma in a needle core biopsy specimen. The myoepithelium may become noticeably attenuated and focally undetectable by immunostains in regions of sclerosis in a papilloma. This finding, by itself, is not diagnostic of carcinoma in the lesion. The epithelial cells in papillomas typically exhibit strong nuclear immunoreactivity for estrogen receptor.
(Fig. 4.11). When immunostains are done to detect myoepithelial cells, it is necessary to distinguish between stromal and myoepithelial reactivity. Fibrous sclerosis can be so severe that it virtually obliterates the papilloma, reducing it to a nodular scar containing scattered, benign glandular elements that may be difficult to distinguish from a fibroadenoma in a needle core biopsy specimen. The myoepithelium may become noticeably attenuated and focally undetectable by immunostains in regions of sclerosis in a papilloma. This finding, by itself, is not diagnostic of carcinoma in the lesion. The epithelial cells in papillomas typically exhibit strong nuclear immunoreactivity for estrogen receptor.
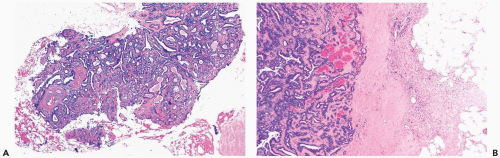
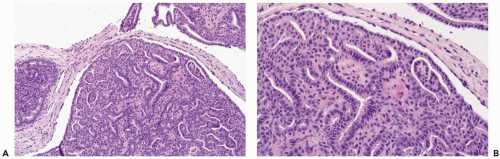 Figure 4.8 Solid papilloma. A, B. Needle core biopsy samples show multinodular and circumscribed architecture. Epithelial hyperplasia is evident, filling the spaces between fibrovascular cores. |
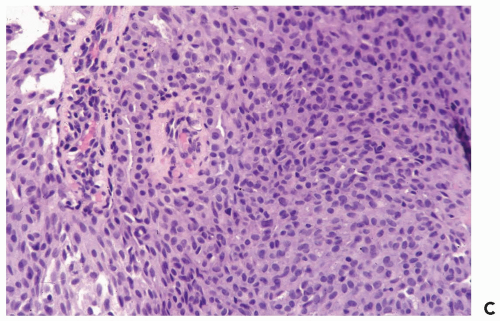 Figure 4.8 (continued) C. An area in another biopsy sample with solid epithelial hyperplasia and inconspicuous fibrovascular cores. |
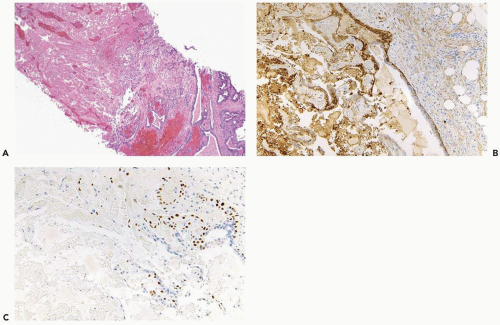
The epithelium of intraductal papillomas contains a myoepithelial cell layer. Myoepithelial cells are not equally apparent in all portions of a papilloma, and they may be focally absent. The nuclei of quiescent myoepithelial cells are usually inconspicuous and flattened along the basement membrane, whereas hyperplastic myoepithelial cells form a prominent layer of columnar or cuboidal cells that often have relatively clear cytoplasm (Fig. 4.12). Some papillomas
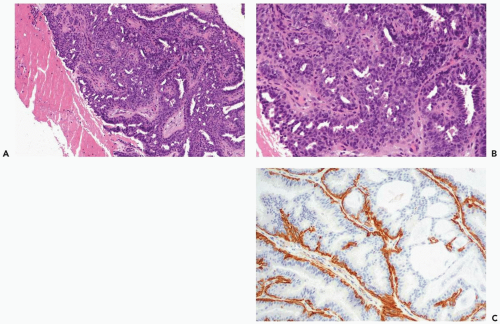
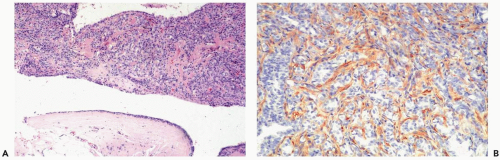
have markedly hyperplastic myoepithelial cells that assume an epithelioid phenotype (Fig. 4.13). Myoepithelial cells are immunoreactive for CK5, actin, calponin, myosin, CD10, and p63 (3) (Fig. 4.14). The p63 immunostain is localized to myoepithelial cell nuclei and infrequently in epithelial nuclei, but it is not reactive with stromal myofibroblasts or vascular structures (4). It is recommended that p63 and at least two other immunostains with cytoplasmic localization be used to compensate for problems such as stromal reactivity or indeterminate staining of one reagent.
have markedly hyperplastic myoepithelial cells that assume an epithelioid phenotype (Fig. 4.13). Myoepithelial cells are immunoreactive for CK5, actin, calponin, myosin, CD10, and p63 (3) (Fig. 4.14). The p63 immunostain is localized to myoepithelial cell nuclei and infrequently in epithelial nuclei, but it is not reactive with stromal myofibroblasts or vascular structures (4). It is recommended that p63 and at least two other immunostains with cytoplasmic localization be used to compensate for problems such as stromal reactivity or indeterminate staining of one reagent.
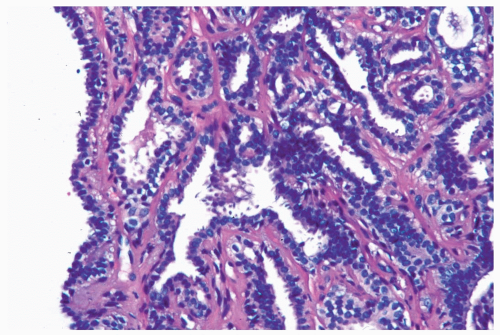 Figure 4.12 Papilloma with prominent myoepithelial cells. Myoepithelial cells with clear cytoplasm are shown outlining glands in this needle core biopsy sample from a papilloma. |
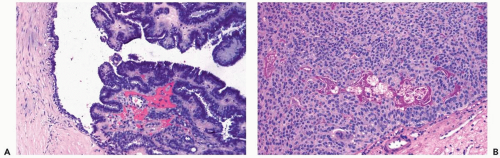
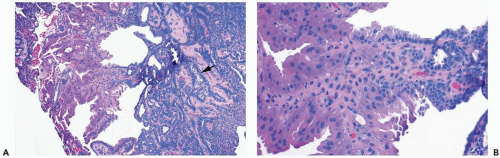
Infarction can occur in a papilloma, often without an apparent extrinsic cause (Fig. 4.15). Chronic inflammation and hemosiderin in and around many papillomas suggest that these lesions are prone to intermittent, transient bleeding. Rarely, the entire lesion is infarcted spontaneously or after a needle biopsy procedure. The underlying architecture of an infarcted papilloma can be demonstrated with a reticulin stain in many instances. Sometimes immunoreactivity for cytokeratin and p63 is preserved in infarcted portions of a papilloma (5). If myoepithelium can be demonstrated in the infarcted tissue, the tumor is more likely a papilloma than a papillary carcinoma. Cytologic atypia, manifested by nuclear hyperchromasia, mitoses and pleomorphism, is commonly found in the partially degenerated epithelium of a papilloma in the vicinity of an infarct. These cytologic abnormalities may lead to an erroneous diagnosis of carcinoma in the needle core biopsy sample from such a lesion.
Squamous metaplasia can occur in the epithelium of a papilloma with infarction or in the absence of infarction (6) (Fig. 4.16). Extension of squamous metaplasia to the epithelium of adjacent ducts is an uncommon finding (7). Entrapped metaplastic epithelium in the stromal reaction may simulate metaplastic or squamous carcinoma, and, in some instances, the distinction between these processes is very difficult (6). However, most papillary tumors of the breast in which there is squamous metaplasia are papillomas.
A series of 26 papillary tumors diagnosed by needle core biopsy included 19 papillomas (8). Ten papillomas were classified as atypical. Calcifications were detected more often in lesions classified as atypical than in papillomas without atypia, whereas a mass was less frequently the mammographic abnormality that led to biopsy of atypical papillomas. Intraductal carcinoma was found in excisional biopsies 3 of 10 (33%) atypical papillomas but not in 7 excised papillomas lacking atypia. At a later date, one of the patients with a papilloma that had no atypia, who did not undergo excisional biopsy, developed invasive carcinoma that arose from residual papilloma.
Several additional studies have correlated the results of core biopsies and subsequent excisional biopsies of papillary lesions. Puglisi et al. (9) reported that core biopsies from 5 of 19 lesions proven by excisional biopsy to be papillary carcinomas were interpreted as showing “absent” or “low” “suspicion of malignancy.” These authors concluded that the sensitivity of the core biopsy procedure was too low to be reliable for excluding carcinoma and that excisional biopsy should be performed. Ivan et al. (10) described 30 patients reported to have a papilloma in a core biopsy. Excision in 6 cases (20%) revealed papilloma without atypia.
The remaining patients had “no clinical or radiologic evidence of disease progression” after follow-up of 1 to 51 months or a mean of 14.7 months. Philpotts et al. (11) studied 16 patients with papillary lesions that were interpreted as benign in needle core biopsy samples. Six patients underwent surgical excision that revealed intraductal carcinoma in one (17%). Among the 10 who did not have an excisional biopsy, follow-up by mammography for 6 to 34 months documented that the lesions were radiologically “gone” in 5 cases, “unchanged” in 2, and “decreased” in 3. In a fourth study, Mercado et al. (12) investigated 12 patients with papillary tumors diagnosed as benign by needle core biopsy. Five of the six who had an excisional biopsy proved to be benign or had no residual lesion, and one (17%) was found to be the site of intraductal carcinoma. Mammographic follow-up in the other six cases revealed decreased size in five and no residual lesion in one case. Excisional biopsies following a diagnosis of papilloma without atypia in 38 consecutive needle core biopsy specimens revealed intraductal carcinoma in 3 cases (8%) studied by Jacobs et al. (13). The intraductal carcinoma was in or near to the excised residual papillary lesion. Jaffer et al. (14) studied excisional biopsies from 99 patients who had an intraductal papilloma diagnosed in a needle core biopsy specimen and reported finding carcinoma in 8.4% (intraductal, 5; invasive, 3). A larger series was reported by Gendler et al. (15), who identified 152 papillary lesions (2%) among 9,310 consecutive needle core biopsy samples. Excisional biopsy documented in 87 (57%), yielded carcinoma, either in situ or invasive, in 15 patients (17%) and atypical hyperplasia in 16 (18%) of patients who had an excisional biopsy.
The remaining patients had “no clinical or radiologic evidence of disease progression” after follow-up of 1 to 51 months or a mean of 14.7 months. Philpotts et al. (11) studied 16 patients with papillary lesions that were interpreted as benign in needle core biopsy samples. Six patients underwent surgical excision that revealed intraductal carcinoma in one (17%). Among the 10 who did not have an excisional biopsy, follow-up by mammography for 6 to 34 months documented that the lesions were radiologically “gone” in 5 cases, “unchanged” in 2, and “decreased” in 3. In a fourth study, Mercado et al. (12) investigated 12 patients with papillary tumors diagnosed as benign by needle core biopsy. Five of the six who had an excisional biopsy proved to be benign or had no residual lesion, and one (17%) was found to be the site of intraductal carcinoma. Mammographic follow-up in the other six cases revealed decreased size in five and no residual lesion in one case. Excisional biopsies following a diagnosis of papilloma without atypia in 38 consecutive needle core biopsy specimens revealed intraductal carcinoma in 3 cases (8%) studied by Jacobs et al. (13). The intraductal carcinoma was in or near to the excised residual papillary lesion. Jaffer et al. (14) studied excisional biopsies from 99 patients who had an intraductal papilloma diagnosed in a needle core biopsy specimen and reported finding carcinoma in 8.4% (intraductal, 5; invasive, 3). A larger series was reported by Gendler et al. (15), who identified 152 papillary lesions (2%) among 9,310 consecutive needle core biopsy samples. Excisional biopsy documented in 87 (57%), yielded carcinoma, either in situ or invasive, in 15 patients (17%) and atypical hyperplasia in 16 (18%) of patients who had an excisional biopsy.
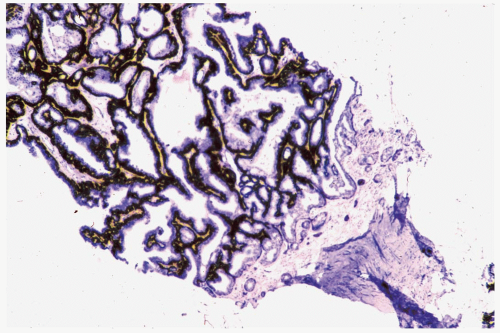 Figure 4.14 Papilloma with myoepithelial cell hyperplasia. An exaggerated myoepithelial cell zone is highlighted by an actin immunostain in this sample (immunoperoxidase: smooth muscle actin). |
Data from the foregoing studies do not provide definitive guidelines for the management of a papilloma without atypia diagnosed by needle core biopsy. A decision as to whether surgical excision should be performed will be influenced by factors, such as lesion size, evidence of persistent tumor post-biopsy, the ease of mammographic follow-up, family history of breast cancer, and patient concerns. In general, surgical excision is recommended for palpable papillomas, and it would be prudent for nonpalpable papillomas when part of the lesion is radiologically evident after the needle core biopsy. Overall, intraductal or invasive carcinoma will be found in 5% to 15% of the excisional biopsy specimens in the absence of atypia in the needle core biopsy sample from a papilloma. Surgical excision should be performed for a papilloma found to have atypical hyperplasia (Figs. 4.17 and 4.18). Agoff and Lawton (16) reported finding in situ or invasive ductal carcinoma in 12 (48%) of 25 patients, with atypical papillary lesions, who underwent an excisional biopsy.
Most follow-up studies of papillomas confirm the low “precancerous” potential of these lesions after complete surgical excision (17,18,19). The reported frequency of carcinoma subsequent to the excision of a papilloma has been less than 5% (20). Macgrogan and Tavossoli (21) described the follow-up of 119 patients who underwent excision of papillary breast tumors. One (4.5%) of 22 women, who had a “papilloma with florid hyperplasia,” developed invasive carcinoma after an interval of 104 months. Among 40 women who had a “papilloma with focal atypia,” 2 (5%) developed invasive carcinoma 35 and 162 months later. Atypical papillomas, defined as lesions in which 10% to 32% of the “papilloma’s entire surface was involved” by atypical epithelium, were found in 24 women. Subsequent carcinomas (2 invasive, 1 intraductal) were diagnosed in 3 (12.5%) after intervals of 28 to 145 months. A greater risk for concurrent (22) or subsequent carcinoma has been demonstrated in women with multiple papillomas than in those with solitary papillomas (19,20,21




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree