OBJECTIVES
After studying this chapter, you should be able to:
Describe hormones and their contribution to whole body homeostatic mechanisms.
Understand the chemical nature of different classes of hormones and how this determines their mechanism of action on target cells.
Define how hormones are synthesized and secreted by cells of endocrine glands, including how peptide hormones are cleaved from longer precursors.
Explain the relevance of protein carriers in the blood for hydrophobic hormones, and the mechanisms that determine the level of free circulating hormones.
Understand the principles of feedback control for hormone release and its relevance for homeostasis.
Understand the principles governing disease states that result from over- or under-production of key hormones.
INTRODUCTION
This section of the text deals with the various endocrine glands that control the function of multiple organ systems of the body. In general, endocrine physiology is concerned with the maintenance of various aspects of homeostasis. The mediators of such control mechanisms are soluble factors known as hormones. The word hormone was derived from the Greek horman, meaning to set in motion. In preparation for specific discussions of the various endocrine systems and their hormones, this chapter will address some concepts of endocrine regulation that are common among all systems.
Another feature of endocrine physiology to keep in mind is that, unlike other physiologic systems that are considered in this text, the endocrine system cannot be cleanly defined along anatomic lines. Rather, the endocrine system is a distributed system of glands and circulating messengers that is often stimulated by the central nervous system or the autonomic nervous system, or both.
EVOLUTION OF HORMONES & THEIR ACTIONS ON TARGET CELLS
As noted in the introduction to this section, hormones comprise steroids, amines, and peptides. Peptide hormones are by far the most numerous. Many hormones can be grouped into families reflecting their structural similarities as well as the similarities of the receptors they activate. However, the number of hormones and their diversity increases as one moves from simple to higher life forms, reflecting the added challenges in providing for homeostasis in more complex organisms. For example, among the peptide hormones, several are heterodimers that share a common α chain, with specificity being conferred by the β-chain. In the specific case of thyroid-stimulating hormone (TSH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH), there is evidence that the distinctive β-chains arose from a series of duplications of a common ancestral gene. For these and other hormones, moreover, this molecular evolution implies that hormone receptors also needed to evolve to allow for spreading of hormone actions/specificity. This was accomplished by co-evolution of the basic G-protein–coupled receptors (GPCR) and receptor tyrosine kinases that mediate the effects of peptide and amine hormones that act at the cell surface (see Chapter 2). The underlying ancestral relationships sometimes reemerge, however, in the cross-reactivity that may be seen when hormones rise to unusually high levels (eg, endocrine tumors).
Steroids and thyroid hormones are distinguished by their predominantly intracellular sites of action, since they can diffuse freely through the cell membrane. They bind to a family of largely cytoplasmic proteins known as nuclear receptors. Upon ligand binding, the receptor–ligand complex translocates to the nucleus where it either homodimerizes, or associates with a distinct liganded nuclear receptor to form a heterodimer. In either case, the dimer binds to DNA to either increase or decrease gene transcription in the target tissue. Individual members of the nuclear receptor family have a considerable degree of homology, perhaps implying a common ancestral gene, and share many functional domains, such as the zinc fingers that permit DNA binding. However, sequence variations allow for ligand specificity as well as binding to specific DNA motifs. In this way, the transcription of distinct genes is regulated by individual hormones.
HORMONE SECRETION
The regulation of hormone synthesis, of course, depends on their chemical nature. For peptide hormones as well as hormone receptors, synthesis is controlled predominantly at the level of transcription. For amine and steroid hormones, synthesis is controlled indirectly by regulating the production of key synthetic enzymes as well as by substrate availability.
Interestingly, the majority of peptide hormones are synthesized initially as much larger polypeptide chains, and then processed intracellularly by specific proteases to yield the final hormone molecule. In some cases, multiple hormones may be derived from the same initial precursor, depending on the specific processing steps present in a given cell type. Presumably this provides for a level of genetic “economy.” It is also notable that the hormone precursors themselves are typically inactive. This may be a mechanism that provides for an additional measure of regulatory control, or, in the case of thyroid hormones, may dictate the site of highest hormone availability.
The synthesis of all of the proteins/peptides discussed above is subject to the normal mechanisms of transcriptional control in the cell (see Chapter 2). In addition, there is provision for exquisitely specific regulation by other hormones, since the regulatory regions of many peptide hormone genes contain binding motifs for the nuclear receptors discussed above. For example, thyroid hormone directly suppresses TSH expression via the thyroid hormone receptor. These specific mechanisms to regulate hormone transcription are essential to the function of feedback loops, as will be addressed in greater detail below. In some cases, the abundance of selected hormones may also be regulated via effects on translation. For example, elevated levels of circulating glucose stimulate the translation of insulin mRNA. These effects are mediated by the ability of glucose to increase the interaction of the insulin mRNA with specific RNA binding proteins, which increase its stability and enhance its translation. The net effect is a more precise and timely regulation of insulin levels, and thus energy metabolism, than could be accomplished with transcriptional regulation alone.
The precursors for peptide hormones are processed through the cellular machinery that handles proteins destined for export, including trafficking through specific vesicles where the propeptide form can be cleaved to the final active hormones. Mature hormones are also subjected to a variety of posttranslational processing steps, such as glycosylation, which can influence their ultimate biologic activity and/or stability in the circulation. Ultimately, all hormones enter either the constitutive or regulated secretory pathway (see Chapter 2).
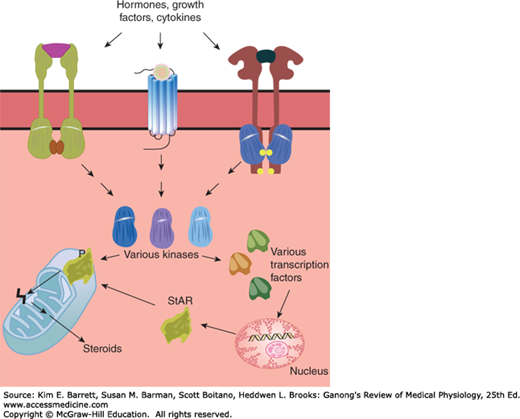
The secretion of many hormones is via a process of exocytosis of stored granules, as discussed in Chapter 2. The exocytotic machinery is activated when the cell type that synthesizes and stores the hormone in question is activated by a specific signal, such as a neurotransmitter or peptide releasing factor. One should, however, contrast the secretion of stored hormones with that of those that are continually released by diffusion (eg, steroids). Control of the secretion of the latter molecules occurs via kinetic influences on the synthetic enzymes or carrier proteins involved in hormone production. For example, the steroidogenic acute regulatory protein (StAR) is a labile protein whose expression, activation, and deactivation are regulated by intracellular signaling cascades and their effectors, including a variety of protein kinases and phosphatases. StAR traffics cholesterol from the outer to the inner membrane leaflet of the mitochondrion. Because this is a rate-limiting first step in the synthesis of the steroid precursor, pregnenolone, this arrangement permits changes in the rate of steroid synthesis, and thus secretion, in response to homeostatic cues such as trophic hormones, cytokines, and stress (Figure 16–1).
FIGURE 16–1
Regulation of steroid biosynthesis by the steroidogenic acute regulatory protein (StAR). Extracellular signals activate intracellular kinases that, in turn, phosphorylate transcription factors that upregulate StAR expression. StAR is activated by phosphorylation, and facilitates transfer of cholesterol from the outer to inner mitochondrial membrane leaflet. This then allows conversion of cholesterol into pregnenolone, which is the first intermediate in the steroid biosynthetic pathway.
An additional complexity related to hormone secretion relates to the fact that some hormones are secreted in a pulsatile manner. Secretion rates may peak and ebb relative to circadian rhythms, in response to the timing of meals, or as regulated by other pattern generators whose periodicity may range from milliseconds to years. Pulsatile secretion is often related to the activity of oscillators in the hypothalamus that regulate the membrane potential of neurons, in turn secreting bursts of hormone releasing factors into the hypophysial blood flow that then cause the release of pituitary and other downstream hormones in a similar pulsatile manner (see Chapters 17 and 18). There is evidence that these hormone pulses convey different information to the target tissues that they act upon compared to a steady exposure to a single concentration of the hormone. Therapeutically, pulsatile secretion may pose challenges if, due to deficiency, it proves necessary to replace a particular hormone that is normally secreted in this way.
HORMONE TRANSPORT IN THE BLOOD
In addition to the rate of secretion and its nature (steady vs. pulsatile), a number of factors influence the circulating levels of hormones. These include the rates of hormone degradation and/or uptake, receptor binding and availability of receptors, and the affinity of a given hormone for plasma carriers (Figure 16–2). Stability influences the circulating half-life of a given hormone and has therapeutic implications for hormone replacement therapy, in addition to those posed by pulsatile secretion as discussed above.