http://evolve.elsevier.com/Edmunds/NP/
Six classes of medications that work via different mechanisms of action are used in the treatment of patients with asthma and COPD. Each of these six groups is discussed in detail as part of the treatment of asthma or COPD. These drugs are also used in the treatment of other respiratory disorders that cause inflammation and bronchospasm. These drugs are largely delivered via inhalation and are designated as long-term controller medications (e.g., inhaled corticosteroids, long-acting inhaled β2-agonists, leukotriene modifiers, theophylline, mast cell stabilizers) and quick relief and other medications (e.g., rapid-acting inhaled β2-agonists, oral corticosteroids, anticholinergics). The nurse practitioner should help design a treatment plan for the patient that includes the components of therapy and provides direction for the treatment of symptom exacerbations. Detailed and ongoing patient education is required for adequate management of these diseases.
The standards of care for patients with asthma were developed by the National Asthma Education and Prevention Program (NAEPP) and were most recently updated in the Expert Panel Report 3 (EPR3). Additional guidelines have been put forth by the Global Initiative for Asthma, Global Strategy for Asthma Management and Prevention, revised in 2006. Both sets of recommendations are based on a step approach that is bidirectional. Guidelines for standard of care management of COPD can be found in the National Heart, Lung, and Blood Institute (NHLBI)–World Health Organization (WHO) GOLD, Global Strategies for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2006).
Asthma and COPD are similar in their chronicity and in terms of their obstructive component. However, asthma differs from COPD in that asthma is largely an inflammatory condition, and it has a greater degree of reversibility than COPD. Drug therapy has not been shown to alter the long-term decline in lung function that occurs in COPD but should be used to control symptoms. Therapy in COPD is also based on a step approach but is unidirectional and cumulative. Although the same drugs are used in treatment for these disorders, asthma and COPD are discussed separately because responses to pharmacotherapy differ.
Therapeutic Overview of Asthma
The respiratory bronchiole is surrounded by smooth muscle and is lined by pseudostratified columnar epithelium containing mucus-secreting goblet cells and cilia. The smooth muscle is innervated by the autonomic nervous system. Parasympathetic stimulation through the vagus nerve and cholinergic receptors in smooth muscle promotes bronchial constriction. Sympathetic stimulation through the action of catecholamines such as epinephrine on β2-adrenergic receptors causes bronchodilation. When the need for airflow is increased, such as with exercise, sympathetic stimulation causes bronchodilation, and opposing parasympathetic bronchoconstrictor tone is inhibited.
The lungs also contain α-adrenergic receptors, and their stimulation results in mild bronchoconstriction. No β1-adrenergic receptors are present in the lungs. β1-adrenergic receptors are the predominant adrenergic receptors of the heart, and their stimulation promotes myocardial contractility and conduction, resulting in an increased heart rate (Table 16-2).
TABLE 16-2
Location and Response of Adrenoreceptors
| Location | Type | Example of Stimulus Response |
| Lung (smooth muscle) | α | Mild bronchoconstriction |
| β2 | Bronchodilation; dilates arteries; relaxes alveolar walls | |
| Mast cells | α | Augments release of histamine and other inflammatory mediators |
| β2 | Inhibits release of inflammatory mediators | |
| Heart | β1 | Increases myocardial contraction, force, and velocity; stimulates glycogenolysis |
| Blood vessels | α | Vasoconstriction |
| β2 | Vasodilation | |
| Skeletal muscle | β2 | Tremor; stimulates glycogenolysis |
| Multiple sites of action | α | Stimulates glycogenolysis; inhibits norepinephrine and acetylcholine release |
| β1 | Stimulates lipolysis | |
| β2 | Stimulates norepinephrine release; inhibits acetylcholine release; moves potassium into cells; stimulates insulin release | |
| Eyes (smooth muscle) | α | Mydriasis |
Modified from Lees GM: A hitchhiker’s guide to the galaxy of adrenoreceptors, BMJ 283:173-178, 1981.
Pathophysiology
The key pathophysiologic features of asthma include (1) airway hyperresponsiveness, (2) airway inflammation, and (3) airway obstruction that is largely, but not always, completely reversible.
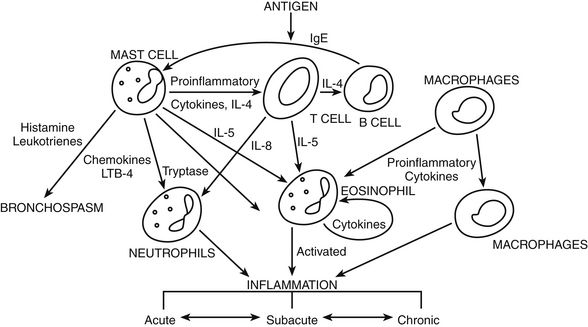
FIGURE 16-1 Cellular mechanisms involved in airway inflammation. From National Asthma Education and Prevention Program Expert Panel Report 2: Guidelines for the diagnosis and management of asthma, Washington, DC, 1997, U.S. Government Printing Office.
Disease Process
Asthma is the result of complex interactions among inflammatory cells, chemical mediators, and associated changes in the airways. Asthma is classified based on symptomatology, function, risk for exacerbation, and pulmonary function; this classification forms a basis for step therapy. Pulmonary function is determined by comparison of the patient’s FEV1 and forced vital capacity (FVC) vs. predicted averages for the patient’s age, height, and gender. Airflow during exhalation is decreased when the airways are narrowed or blocked. Peak expiratory flow (PEF) monitoring can be used by many patients to monitor their lung function at home. This allows them to anticipate when their breathing will become worse and to take appropriate medications or call their health care provider before symptoms become too severe. (Note: Slight variations in the following classification criteria have been defined for children younger than 12; see website for additional information.)
A patient need only meet one criterion to move into a new severity rating; thus, patients should be reevaluated frequently to determine whether the severity has increased or decreased. Signs of worsening asthma may include a decrease in peak flow, increased cough, a greater degree of breathlessness, the occurrence of wheezing, particularly at night, and increased chest tightness. Use of accessory muscles of respiration and suprasternal retractions indicate severe exacerbation. Patients may also complain of chest tightness and productive or nonproductive cough. The chest should be assessed for use of accessory muscles, wheezing, and prolonged forced expiration. Wheezing may be absent between episodes of asthma, as well as during severe airflow limitation.
Asthma is difficult to diagnose in infants and children younger than 5. Viral respiratory infection is the most common cause of asthma symptoms in this age group.
Therapeutic Overview of Chronic Obstructive Pulmonary Disease
Inspired air moves through conducting airways as it moves from the environment to the lungs for gas exchange. Conducting airways warm and humidify air and remove foreign material through mucus-secreting goblet cells and ciliary projections in the columnar epithelium. Mucociliary clearance is enhanced by optimal oxygenation, humidification, and coughing. Airway clearance is impaired by low or high oxygen levels, dryness, and cigarette smoking. Alveoli are thin-walled sacs separated by septa and surrounded by a network of capillaries to facilitate gas exchange. Diffusion of gases across the alveolar capillary membrane can be affected by concentration of inspired oxygen; condition of the lung tissue, which affects the surface area available for diffusion; and thickness of the alveolar capillary membrane.
Pathophysiology
Several mechanisms are involved in the pathogenesis of COPD, including airway inflammation, edema, fibrosis of the bronchial wall, hypertrophy of the submucosal glands, hypersecretion of mucus, loss of elastic lung recoil, and destruction of alveolar tissue. Tobacco smoking and other noxious stimuli produce an inflammatory response throughout the lungs, including the parenchyma and vasculature. Inflammatory cells invade the damaged lung tissue and secrete numerous mediators, including tumor necrosis factor (TNF), interleukin-8, and leukotriene B4 (LTB4). The larger airways (e.g., trachea, bronchi, and bronchioles >2 mm) respond by increasing the number of mucus-secreting glands and reducing mucociliary function. The smaller airways (bronchioles <2 mm) go through repeated cycles of injury and repair, resulting in remodeling, scarring, thickening, and consolidation. This results in increased airflow resistance, as evidenced by decreased FEV1 and FEV1/FVC measurements.
As lung parenchyma is destroyed, elastic recoil is lost, thus reducing the force of expiratory airflow and resulting in hyperinflation. Hyperinflation leads to flattening of the diaphragm, an increase in the anteroposterior diameter of the chest (“barrel chest”), and reduced respiratory muscle strength. The loss of alveoli decreases the surface area available for gas exchange. Changes in the vasculature of the lungs caused by the remodeling process can eventually lead to pulmonary hypertension, cor pulmonale, and severe hypoxia and hypercapnia.
Disease Process
COPD is a disease state characterized by airflow limitation that is not fully reversible. Airflow limitation usually is progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases. Although symptoms of COPD vary throughout the illness, they are not a good predictor of degree of airflow limitation. Therefore, COPD has been reclassified by some authors according to spirometry. Predicted values are calculated for each individual patient. The four stages of severity of COPD are as follows:
Any stage of COPD may be marked by exacerbation of varying severity, but exacerbation is more common in those patients with FEV1 <50% of predicted value. Many exacerbations are due to respiratory infection or exposure to air pollution, but in about one half of cases, the cause of exacerbation cannot be identified.
The earliest symptom of COPD is often morning cough with sputum that is clear to yellow. Frequent respiratory infections increase cough, often turn the sputum yellow or green, and result in periods of wheezing. Later, shortness of breath develops with exertion and becomes progressively more severe. On auscultation, expiration may be prolonged, expiratory wheezing is often present, and crackles may be audible.
Spirometry is the gold standard by which the diagnosis of COPD should be established and disease severity determined. FEV1 and FEV1/FVC are determined before and after bronchodilator therapy to make the diagnosis and determine severity. COPD causes a progressive decline in FEV1, FVC, and FEV1/FVC measurements that is greater than would be expected with an age-related decline in lung function.
An arterial blood gas measurement is recommended if FEV1 is <50% of predicted value. Pulse oximetry is more easily carried out to measure oxygen (O2) saturation in the blood and can be used for monitoring during rest, activity, and sleep. A chest x-ray film will show hyperinflation of the lungs and flattening of the diaphragm in advanced COPD. These tests are necessary to confirm the correct diagnosis and to rule out other types of respiratory disease.
Mechanism of Action
β-Adrenergic Agonist Bronchodilators
β-adrenergic agonists are sympathomimetics. The basic action of β-agonists is to activate the enzyme adenyl cyclase, which increases the production of cyclic adenosine monophosphate (cAMP). Intracellular cAMP inhibits phosphorylation of myosin and lowers intracellular concentrations of calcium. The result is smooth muscle relaxation. Bronchodilation reduces airway resistance as shown by increased FEV1, mid-expiratory flow rate, and vital capacity. Increased cAMP also inhibits the release of mediators from mast cells in the airways, thereby producing a mild antiinflammatory effect.
Nonselective β2-adrenergic receptor agonists and, to a lesser extent, selective β2-adrenergic agonists cause reflex tachycardia. This produces arterial dilation that results from β2-receptor stimulation of the heart. Albuterol and the other bronchodilators that are relatively β2 selective have their greatest effect on β-adrenergic receptors in the bronchial, uterine, and vascular smooth muscles. Bronchodilators developed to date are only relatively selective in stimulating β2-receptors, and all have varying incidences of cardiac side effects and muscle tremor. At higher doses, the selective drugs may lose their receptor selectivity and cause β1 stimulation.
The role of rapid-acting inhaled β2-agonists (RABAs; also known as short-acting β2-agonists [SABAs]) in asthma therapy is to provide relief of bronchospasm during exacerbation or pretreatment before exercise. RABAs and long-acting β2-agonists (LABAs) are considered first-line drugs in the treatment of both asthma and COPD. Increased use of RABAs may signal the need for additional drug therapy. Inability to achieve an adequate response with a β2-agonist during an exacerbation may indicate the need for the addition of a short-term corticosteroid (inhaled or oral).
Methylxanthines
Methylxanthines promote bronchodilation by competitively inhibiting phosphodiesterase, the enzyme that degrades cAMP, which in turn increases intracellular cAMP. (See β-Adrenergic Agonist Bronchodilators [above] for the action of increased cAMP.) Methylxanthines also act as direct central nervous system stimulants that produce vasoconstriction and stimulation of the vagal center, which causes bradycardia.
Methylxanthines in large doses have a positive inotropic effect on the myocardium and a positive chronotropic effect on the sinoatrial node, causing transient increases in heart rate, force of contraction, cardiac output, and myocardial oxygen demand. At high concentrations, vagal stimulation is masked by increased sinus rate and may result in hypotension, extrasystole, and arrhythmia. Additional effects of methylxanthines include diuresis through dilation of renal arterioles, increased cardiac output, and inhibition of reabsorption of sodium and potassium in the proximal renal tubules. The gastrointestinal system is affected by relaxation of smooth muscle, causing reduced lower esophageal sphincter pressure and relaxed biliary contraction, along with stimulation of gastric secretions. The role of theophylline in the control of asthma and COPD is not a primary one because studies have shown that it is not as effective as inhaled LABAs or corticosteroids. It may be beneficial in some cases when given as add-on therapy.
Anticholinergics
Similar to atropine, anticholinergics are nonselective competitive antagonists of muscarinic receptors present in the airways and other organs. The anticholinergic drug blocks acetylcholine-induced stimulation of cyclic guanyl cyclase, thereby reducing production of cyclic guanosine monophosphate (cGMP), a mediator of bronchoconstriction. Airway secretions are reduced by these drugs. Airway resistance also is reduced, as measured by increases in FEV1 and the middle portion of forced expiratory flow (FEF25-75). Ipratropium and tiotropium when inhaled may be more effective in COPD than in asthma because it is believed that cholinergic tone of the airways is increased in COPD.
Ipratropium exhibits greater antimuscarinic effect on bronchial smooth muscle than on secretory glands, especially with oral inhalation of the drug. Additional antimuscarinic effects may include mydriasis, inhibition of salivary and gastric secretions, tachycardia, and spasmolysis; however, clinical trials have shown these effects to be insignificant in healthy individuals and in those with COPD. Anticholinergics often are used first line as an adjunct or alternative to β2-agonist therapy to avoid β2 side effects, and because they have a longer duration of effect.
Mast Cell Stabilizers
Mast cell stabilizers prevent and reduce the inflammatory response in bronchial walls by inhibiting the secretion of mediators from mast cells.
The exact mechanism of action of these drugs on mast cells remains to be established. Medications in this class act locally to inhibit the release of mediators of type 1 allergic reactions, including histamine and leukotrienes, from sensitized mast cells following exposure to an antigen. They inhibit type III (late) inflammatory reactions to a lesser extent. These drugs are antiasthmatic and antiallergic, and they also may act as bronchodilators.
Corticosteroids
Corticosteroids are hormonal agents that have a profound antiinflammatory effect. Corticosteroids reduce airflow obstruction by reducing airway inflammation in the bronchioles.
Corticosteroids modify the body’s immune responses to various stimuli. They suppress cytokine production, airway eosinophil recruitment, and the release of inflammatory mediators. Inhaled corticosteroids (ICSs) provide local therapeutic action with minimal systemic effects. (See Chapter 51 for a detailed discussion of mechanism of action.) ICSs have been shown to reduce asthma symptoms, improve quality of life, improve lung function, decrease airway hyperresponsiveness, control airway inflammation, reduce frequency and severity of exacerbations, and reduce asthma mortality. Oral corticosteroid treatment often is indicated for exacerbation of asthma and some cases of COPD. ICS therapy in COPD does not alter the course of the disease but does control symptoms and has been shown to reduce the frequency of exacerbations.
Leukotriene Modifiers
Leukotriene modifiers act on inflammatory mediators of asthma, the LTs (also known as slow-reacting substance of anaphylaxis [SRS-A]), which contribute to airway obstruction.
Cysteinyl LTs (cysLTs) are more potent and longer-acting bronchoconstrictors than histamine. They are produced in a variety of cells (e.g., eosinophils, mast cells, basophils, macrophages, and monocytes) from arachidonic acid through several enzyme pathways (Figure 16-2). Arachidonic acid is released from cell membranes in response to antigen–antibody reactions, immunoglobulin (Ig)E receptor activation, microorganisms, and physical stimuli. LTs enhance responsiveness of the airways to a variety of stimuli and stimulate secretion of mucus in the airways. Zafirlukast and montelukast are potent competitive leukotriene receptor antagonists (LTRAs). They block the action of cysLTs at receptor sites on smooth muscle cells throughout the bronchioles. A single type of receptor is able to mediate profound contractions brought about by the cysLTs. LTRAs prevent the binding of LTs to their receptors in the airways, thereby blocking bronchospasm. Zileuton is a 5-lipoxygenase (5-LO) pathway inhibitor. 5-LO is one of the pathways through which arachidonic acid can be metabolized to form LTs. The effect of both types of drugs is inhibition of the acute bronchoconstriction phase and inhibition of the delayed inflammatory phase of asthma. Bronchodilation with improved FEV1 and reduced markers of airway inflammation (particularly eosinophils) is the result. Leukotriene modifiers (LMs) may be used as an alternative to ICSs in patients with mild persistent or aspirin-sensitive asthma, but their effect is weaker than that of low-dose ICSs; therefore, they usually are used as add-on therapy in asthma.
Treatment Principles of Asthma
For asthma: Several current guidelines have been recommended for asthma management. The first came from the National Asthma Education and Prevention Program (NAEPP) out of the NHLBI in 1991. These guidelines were updated in 1997, 2002, and, most recently, in 2007, in the NAEPP EPR3. The NAEPP guidelines present a step therapy approach to asthma management. An additional guideline came from the Global Initiative for Asthma (GINA), revised in 2006. GINA is a result of collaboration of NHLBI and WHO for the purpose of setting up a network to disseminate information on asthma management around the world and incorporating results of scientific trials pertaining to asthma management. Both guidelines contain detailed information on diagnosis, assessment, pharmacologic management, and patient education for implementation of individual asthma treatment plans. The recommendations of both guidelines are presented in this chapter. Additional guidelines that specifically address optimal asthma control by focusing on assessment and presenting a modified step approach come from the Joint Task Force on Practice Parameters, 2005; however, because they do not outline medication treatment in detail, these guidelines are not discussed in this chapter.
Evidence-Based Recommendations
Meta-analyses and systematic reviews of clinical trials seek to clarify and prioritize drug treatment for optimal control of asthma. Many clinical trials have sought to clarify the use of ICSs in asthma management in terms of dose, systemic side effects, combination therapy, and possible alternative antiinflammatory drugs. It has been found that corticosteroids exhibit a dose-response relationship for efficacy but at the expense of increasing side effects in the high-dose range. People with mild to moderate asthma gained the most benefit from low to moderate doses of beclomethasone, budesonide, and fluticasone. A small decrease has been found in the initial structural growth of bones in children receiving ICSs at high doses, but no difference was noted in adult height, bone mineral density, or urinary or plasma cortisol levels among children treated with long-term corticosteroids. A review of studies in which ICSs were used vs. the combination of ICSs and LABAs showed the combination of ICSs and LABAs to be more efficacious and cost-effective in terms of direct medical costs, episode-free days, and symptom-free days. The addition of the LABA (salmeterol or formoterol) has been found to be more efficacious in terms of preventing exacerbations and in influencing a number of other outcomes, such as rescue-free days, symptom-free days, quality of life, and symptom scores. Nedocromil showed a good safety profile with no significant short- or long-term side effects, but results regarding its efficacy were conflicting. The combination of the bronchodilators albuterol and ipratropium vs. albuterol alone was studied for efficacy and cost in terms of emergency situations associated with asthma exacerbations. Nebulized albuterol vs. the combination product of albuterol and ipratropium was used in the emergency department. Although the cost of the combination product was greater, it was not more efficacious in terms of PEF rate or rate of admission to the hospital.
Cardinal Points of Treatment
Pharmacologic Treatment


 Top 100 drug;
Top 100 drug;  key drug.
key drug.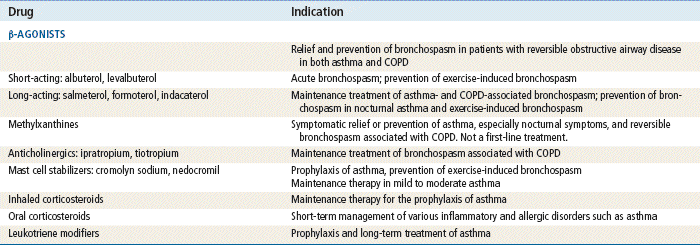

 LABAs used without corticosteroids increase the risk of serious adverse effects and death in asthma. LABAs should never be used without an accompanying corticosteroid.
LABAs used without corticosteroids increase the risk of serious adverse effects and death in asthma. LABAs should never be used without an accompanying corticosteroid.

