236 | Histoplasmosis |
ETIOLOGY
![]() Histoplasma capsulatum, a thermal dimorphic fungus, is the etiologic agent of histoplasmosis. In most endemic areas, H. capsulatum var. capsulatum is the causative agent. In Africa, H. capsulatum var. duboisii also is found; var. duboisii can be differentiated from var. capsulatum as the duboisii yeasts are larger. In Central and South America, histoplasmosis is common and is caused by clades of H. capsulatum var. capsulatum that differ genetically from those involved elsewhere.
Histoplasma capsulatum, a thermal dimorphic fungus, is the etiologic agent of histoplasmosis. In most endemic areas, H. capsulatum var. capsulatum is the causative agent. In Africa, H. capsulatum var. duboisii also is found; var. duboisii can be differentiated from var. capsulatum as the duboisii yeasts are larger. In Central and South America, histoplasmosis is common and is caused by clades of H. capsulatum var. capsulatum that differ genetically from those involved elsewhere.
Mycelia—the naturally infectious form of Histoplasma—have a characteristic appearance, with microconidial and macroconidial forms. Microconidia are oval and are small enough (2–4 μm) to reach the terminal bronchioles and alveoli. Shortly after infecting the host, mycelia transform into the yeasts that are found inside macrophages and other phagocytes. The yeast forms are characteristically small (2–5 μm), with occasional narrow budding. In the laboratory, mycelia are best grown at room temperature, whereas yeasts are grown at 37°C on enriched media.
EPIDEMIOLOGY
![]() Histoplasmosis is the most prevalent endemic mycosis in North America. Although this fungal disease has been reported throughout the world, its endemicity is particularly notable in certain parts of North, Central, and South America; Africa; and Asia. In Europe, histoplasmosis is diagnosed fairly often, mostly in emigrants from or travelers to endemic areas on other continents. In the United States, the endemic areas spread over the Ohio and Mississippi river valleys. This pattern is related to the humid and acidic nature of the soil in these areas. Soil enriched with bird or bat droppings promotes the growth and sporulation of Histoplasma. Disruption of soil containing the organism leads to aerosolization of the microconidia and exposure of humans nearby. Activities associated with high-level exposure include spelunking, excavation, cleaning of chicken coops, demolition and remodeling of old buildings, and cutting of dead trees. Most cases seen outside of highly endemic areas represent imported disease—e.g., cases reported in Europe after travel to the Americas, Africa, or Asia.
Histoplasmosis is the most prevalent endemic mycosis in North America. Although this fungal disease has been reported throughout the world, its endemicity is particularly notable in certain parts of North, Central, and South America; Africa; and Asia. In Europe, histoplasmosis is diagnosed fairly often, mostly in emigrants from or travelers to endemic areas on other continents. In the United States, the endemic areas spread over the Ohio and Mississippi river valleys. This pattern is related to the humid and acidic nature of the soil in these areas. Soil enriched with bird or bat droppings promotes the growth and sporulation of Histoplasma. Disruption of soil containing the organism leads to aerosolization of the microconidia and exposure of humans nearby. Activities associated with high-level exposure include spelunking, excavation, cleaning of chicken coops, demolition and remodeling of old buildings, and cutting of dead trees. Most cases seen outside of highly endemic areas represent imported disease—e.g., cases reported in Europe after travel to the Americas, Africa, or Asia.
PATHOGENESIS AND PATHOLOGY
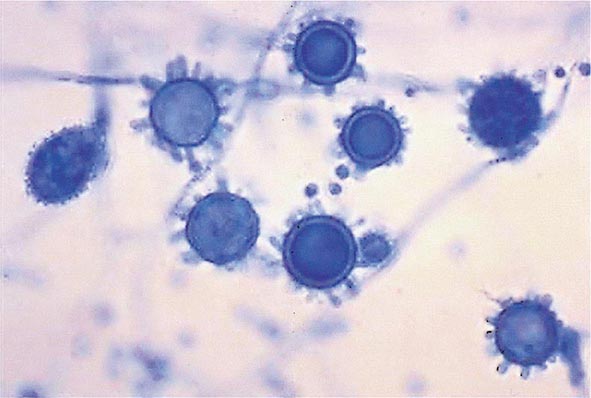
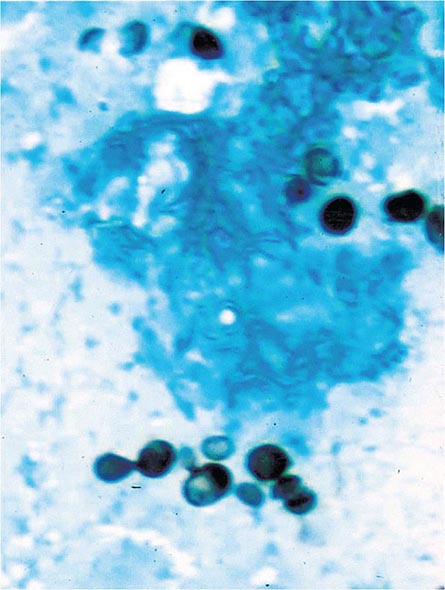
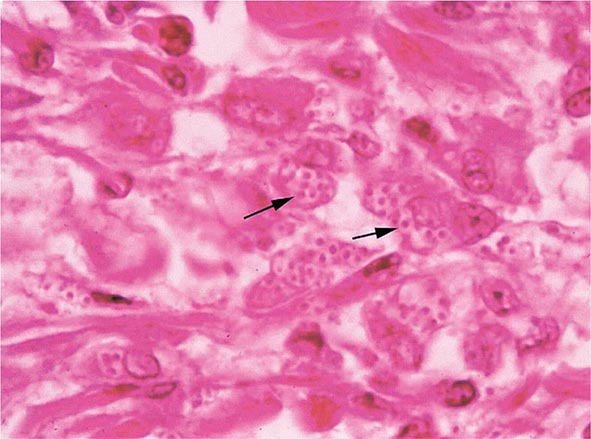
Infection follows inhalation of microconidia (Fig. 236-1). Once they reach the alveolar spaces, microconidia are rapidly recognized and engulfed by alveolar macrophages. At this point, the microconidia transform into budding yeasts (Fig. 236-2), a process that is integral to the pathogenesis of histoplasmosis and is dependent on the availability of calcium and iron inside the phagocytes. The yeasts are capable of growing and multiplying inside resting macrophages. Neutrophils and then lymphocytes are attracted to the site of infection. Before the development of cellular immunity, yeasts use the phagosomes as a vehicle for translocation to local draining lymph nodes, whence they spread hematogenously throughout the reticuloendothelial system. Adequate cellular immunity develops ~2 weeks after infection. T cells produce interferon γ to assist the macrophages in killing the organism and controlling the progression of disease. Interleukin 12 and tumor necrosis factor α (TNF-α) play an essential role in cellular immunity to H. capsulatum. In the immunocompetent host, macrophages, lymphocytes, and epithelial cells eventually organize and form granulomas that contain the organisms. These granulomas typically fibrose and calcify; calcified mediastinal lymph nodes and hepatosplenic calcifications are frequently found in healthy individuals from endemic areas. In immunocompetent hosts, infection with H. capsulatum confers some immunity to reinfection. In patients with impaired cellular immunity, the infection is not contained and can disseminate. Progressive disseminated histoplasmosis (PDH) can involve multiple organs, most commonly the bone marrow, spleen, liver (Fig. 236-3), adrenal glands, and mucocutaneous membranes. Unlike latent tuberculosis, latent histoplasmosis rarely reactivates.
FIGURE 236-1 Spiked spherical conidia of H. capsulatum (lactophenol cotton blue stain).
FIGURE 236-2 Small (2–5 μm) narrow budding yeasts of H. capsulatum from bronchoalveolar lavage fluid (Grocott’s methenamine silver stain).
FIGURE 236-3 Intracellular yeasts (arrows) of H. capsulatum in a liver biopsy specimen (hematoxylin and eosin stain).
Structural lung disease (e.g., emphysema) impairs the clearance of pulmonary histoplasmosis, and chronic pulmonary disease can result. This chronic process is characterized by progressive inflammation, tissue necrosis, and fibrosis mimicking cavitary tuberculosis.
CLINICAL MANIFESTATIONS
The clinical spectrum of histoplasmosis ranges from asymptomatic infection to life-threatening illness. The attack rate and the extent and severity of the disease depend on the intensity of exposure, the immune status of the exposed individual, and the underlying lung architecture of the host.
In immunocompetent individuals with low-level exposure, most Histoplasma infections are either asymptomatic or mild and self-limited. Of adults residing in endemic areas, 50–80% have skin-test and/or radiographic evidence of previous infection without clinical manifestations. When symptoms do develop, they usually appear 1–4 weeks after exposure. Heavy exposure leads to a flulike illness with fever, chills, sweats, headache, myalgia, anorexia, cough, dyspnea, and chest pain. Chest radiographs usually show signs of pneumonitis with prominent hilar or mediastinal adenopathy. Pulmonary infiltrates may be focal with light exposure or diffuse with heavy exposure. Rheumatologic symptoms of arthralgia or arthritis, often associated with erythema nodosum, occur in 5–10% of patients with acute histoplasmosis. Pericarditis may also develop. These manifestations represent inflammatory responses to the acute infection rather than its direct effects. Hilar or mediastinal lymph nodes may undergo necrosis and coalesce to form large mediastinal masses that can cause compression of great vessels, proximal airways, and the esophagus. These necrotic lymph nodes may also rupture and create fistulas between mediastinal structures (e.g., bronchoesophageal fistulas).
PDH is typically seen in immunocompromised individuals, who account for ~70% of cases. Common risk factors include AIDS (CD4+ T cell count, <200/μL), extremes of age, immunosuppressive medications administered for prevention or treatment of rejection following transplantation (e.g., prednisone, mycophenolate, calcineurin inhibitors, and biologic response modifiers), and methotrexate, anti-TNF-α agents, or other biologic response modifiers given for inflammatory arthritis or Crohn’s disease.
The spectrum of PDH ranges from an acute, rapidly fatal course—with diffuse interstitial or reticulonodular lung infiltrates causing respiratory failure, shock, coagulopathy, and multiorgan failure—to a more subacute course with a focal organ distribution. Common manifestations include fever and weight loss. Hepatosplenomegaly also is common. Other findings may include meningitis or focal brain lesions, ulcerations of the oral mucosa, gastrointestinal ulcerations, and adrenal insufficiency. Prompt recognition of this devastating illness is of paramount importance in patients with more severe manifestations or with underlying immunosuppression, especially AIDS (Chap. 226).
Chronic cavitary histoplasmosis is seen in smokers who have structural lung disease (e.g., bullous emphysema). This chronic illness is characterized by productive cough, dyspnea, low-grade fever, night sweats, and weight loss. Chest radiographs usually show upper-lobe infiltrates, cavitation, and pleural thickening—findings resembling those of tuberculosis. Without treatment, the course is slowly progressive.
Fibrosing mediastinitis is an uncommon and serious complication of histoplasmosis. In certain patients, acute infection is followed for unknown reasons by progressive fibrosis around the hilar and mediastinal lymph nodes. Involvement may be unilateral or bilateral; bilateral involvement carries a worse prognosis. Major manifestations include superior vena cava syndrome, obstruction of pulmonary vessels, and airway obstruction. Patients may experience recurrent pneumonia, hemoptysis, or respiratory failure. Fibrosing mediastinitis is fatal in up to one-third of cases.
In healed histoplasmosis, calcified mediastinal nodes or lung parenchyma may erode through the walls of the airways and cause hemoptysis. This condition is called broncholithiasis.
![]() The clinical features and management of histoplasmosis caused by the genetically different clades in Central and South America are similar to those of the disease in North America. African histoplasmosis caused by var. duboisii is clinically distinct and is characterized by frequent skin and bone involvement.
The clinical features and management of histoplasmosis caused by the genetically different clades in Central and South America are similar to those of the disease in North America. African histoplasmosis caused by var. duboisii is clinically distinct and is characterized by frequent skin and bone involvement.
DIAGNOSIS
Fungal culture remains the gold standard diagnostic test for histoplasmosis. However, culture results may not be known for up to 1 month, and cultures are often negative in less severe cases. Cultures are positive in ~75% of cases of PDH and chronic pulmonary histoplasmosis. Cultures of bronchoalveolar lavage (BAL) fluid are positive in about half of cases that include acute pulmonary histoplasmosis causing diffuse infiltrates with hypoxemia. In PDH, the culture yield is highest for BAL fluid, bone marrow aspirate, and blood. Cultures of sputum or bronchial washings are usually positive in chronic pulmonary histoplasmosis. Cultures are typically negative, however, in other forms of histoplasmosis.
Fungal stains of cytopathology or biopsy materials showing structures resembling Histoplasma yeasts are helpful in the diagnosis of PDH, yielding positive results in about half of cases. Yeasts can be seen in BAL fluid (Fig. 236-2) from patients with diffuse pulmonary infiltrates, in bone marrow biopsy samples, and in biopsy specimens of other involved organs (e.g., the adrenal glands). Occasionally, yeasts are seen in blood smears from patients with severe PDH. However, artifacts and other fungal elements sometimes stain positive and may be misidentified as Histoplasma yeasts.
The detection of Histoplasma antigen in body fluids is extremely useful in the diagnosis of PDH and acute diffuse pulmonary histoplasmosis. The sensitivity of this technique is >95% in patients with PDH and >80% in patients with acute pulmonary histoplasmosis if both urine and serum are tested. Antigen level correlates with severity of illness in PDH and can be used to follow disease progression as levels predictably decrease with effective therapy. An increase in antigen levels also predicts relapse. Antigen can be detected in cerebrospinal fluid from patients with meningitis and in BAL fluid from those with pneumonia. Cross-reactivity occurs with African histoplasmosis, blastomycosis, coccidioidomycosis, paracoccidioidomycosis, and Penicillium marneffei infection.
Serologic tests, including immunodiffusion and complement fixation, are useful for the diagnosis of histoplasmosis in immunocompetent patients. Serum antibody titers may rise fourfold in patients with acute histoplasmosis. Serologic tests are especially useful for the diagnosis of chronic pulmonary histoplasmosis. The limitations of serology, however, include insensitivity early in the course of infection (at least 1 month is required for the production of antibodies), insensitivity in immunosuppressed patients, and persistence of detectable antibody for several years after infection. Positive results from past infection may lead to a misdiagnosis of active histoplasmosis in a patient with another disease process.
237 | Coccidioidomycosis |
DEFINITION AND ETIOLOGY
Coccidioidomycosis, commonly known as Valley fever (see “Epidemiology,” below), is caused by dimorphic soil-dwelling fungi of the genus Coccidioides. Genetic analysis has demonstrated the existence of two species, C. immitis and C. posadasii. These species are indistinguishable with regard to the clinical disease they cause and their appearance on routine laboratory media. Thus, the organisms will be referred to simply as Coccidioides for the remainder of this chapter.
EPIDEMIOLOGY
![]() Coccidioidomycosis is confined to the Western Hemisphere between the latitudes of 40°N and 40°S. In the United States, areas of high endemicity include the southern portion of the San Joaquin Valley of California and the south-central region of Arizona. However, infection may be acquired in other areas of the southwestern United States, including the southern coastal counties in California, southern Nevada, southwestern Utah, southern New Mexico, and western Texas, including the Rio Grande Valley. Outside the United States, coccidioidomycosis is endemic to northern Mexico as well as to localized regions of Central America. In South America, there are endemic foci in Colombia, Venezuela, northeastern Brazil, Paraguay, Bolivia, and north-central Argentina.
Coccidioidomycosis is confined to the Western Hemisphere between the latitudes of 40°N and 40°S. In the United States, areas of high endemicity include the southern portion of the San Joaquin Valley of California and the south-central region of Arizona. However, infection may be acquired in other areas of the southwestern United States, including the southern coastal counties in California, southern Nevada, southwestern Utah, southern New Mexico, and western Texas, including the Rio Grande Valley. Outside the United States, coccidioidomycosis is endemic to northern Mexico as well as to localized regions of Central America. In South America, there are endemic foci in Colombia, Venezuela, northeastern Brazil, Paraguay, Bolivia, and north-central Argentina.
The risk of infection is increased by direct exposure to soil harboring Coccidioides. Because of difficulty in isolating Coccidioides from the soil, the precise characteristics of potentially infectious soil are not known. In the United States, several outbreaks of coccidioidomycosis have been associated with soil from archaeologic excavations of Amerindian sites both within and outside of the recognized endemic region. These cases often involved alluvial soils in regions of relative aridity with moderate temperature ranges. Coccidioides was isolated at depths of 2–20 cm below the surface. The recent identification of three cases of coccidioidomycosis in eastern Washington State may suggest that the endemic region is expanding.
In endemic areas, many cases of Coccidioides infection occur without obvious soil or dust exposure. Climatic factors appear to increase the infection rate in these regions. In particular, periods of aridity following rainy seasons have been associated with marked increases in the number of symptomatic cases. Overall, the incidence within the United States has increased substantially over the past decade, with nearly 43 cases per 100,000 residents of the endemic region in 2011. Most of that increase has occurred in south-central Arizona, where most of that state’s population resides, and in the southern San Joaquin Valley of California, a much less populated region. The factors causing this increase have not been fully elucidated; however, an influx of older individuals without prior coccidioidal infection appears to be involved. Other variables, such as climate change, construction activity, and increased awareness and reporting, may also be factors. Health care providers should consider coccidioidomycosis when evaluating persons with pneumonia who live in or have traveled to endemic areas.
PATHOGENESIS, PATHOLOGY, AND IMMUNE RESPONSE
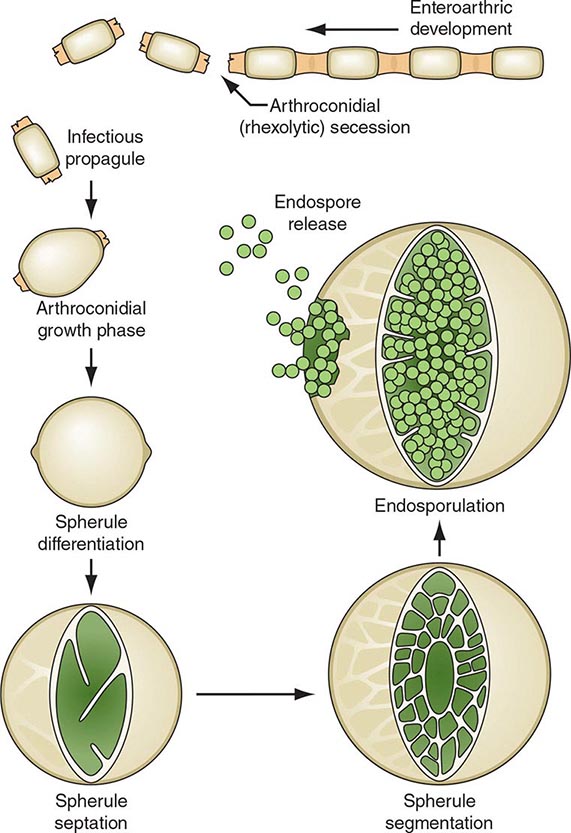
On agar media and in the soil, Coccidioides organisms exist as filamentous molds. Within this mycelial structure, individual filaments (hyphae) elongate and branch, some growing upward. Alternating cells within the hyphae degenerate, leaving barrel-shaped viable elements called arthroconidia. Measuring ∼2 by 5 μm, arthroconidia may become airborne for extended periods. Their small size allows them to evade initial mechanical mucosal defenses and reach deep into the bronchial tree, where infection is initiated in the nonimmune host.
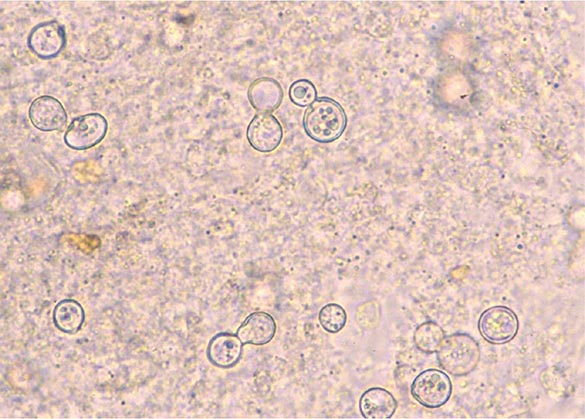
Once in a susceptible host, the arthroconidia enlarge, become rounded, and develop internal septations. The resulting structures, called spherules (Fig. 237-1), may attain sizes of 200 μm and are unique to Coccidioides. The septations encompass uninuclear elements called endospores. Spherules may rupture and release packets of endospores that can themselves develop into spherules, thus propagating infection locally. If returned to artificial media or the soil, the fungus reverts to its mycelial stage.
FIGURE 237-1 Life cycle of Coccidioides. (From TN Kirkland, J Fierer: Emerg Infect Dis 2:192, 1996.)
Clinical observations and data from studies of animals strongly support the critical role of a robust cellular immune response in the host’s control of coccidioidomycosis. Necrotizing granulomas containing spherules are typically identified in patients with resolved pulmonary infection. In disseminated disease, granulomas are generally poorly formed or do not develop at all, and a polymorphonuclear leukocyte response occurs frequently. In patients who are asymptomatic or in whom the initial pulmonary infection resolves, delayed-type hypersensitivity to coccidioidal antigens has been routinely documented.
CLINICAL AND LABORATORY MANIFESTATIONS
Of infected individuals, 60% are completely asymptomatic, and the remaining 40% have symptoms that are related principally to pulmonary infection, including fever, cough, and pleuritic chest pain. The risk of symptomatic illness increases with age. Coccidioidomycosis is commonly misdiagnosed as community-acquired bacterial pneumonia.
There are several cutaneous manifestations of primary pulmonary coccidioidomycosis. Toxic erythema consisting of a maculopapular rash has been noted in some cases. Erythema nodosum (see Fig. 25e-40)—typically over the lower extremities—or erythema multiforme (see Fig. 25e-25)—usually in a necklace distribution—may occur; these manifestations are seen particularly often in women. Arthralgias and arthritis may develop. The diagnosis of primary pulmonary coccidioidomycosis is suggested by a history of night sweats or profound fatigue as well as by peripheral-blood eosinophilia and hilar or mediastinal lymphadenopathy on chest radiography. While pleuritic chest pain is common, pleural effusions occur in fewer than 10% of cases. Such effusions are invariably associated with a pulmonary infiltrate on the same side. The cellular content of these effusions is mononuclear in nature; Coccidioides is rarely grown from effusions.
In most patients, primary pulmonary coccidioidomycosis usually resolves without sequelae in weeks. However, several pneumonic complications may arise. Pulmonary nodules are residua of primary pneumonia. Generally single, frequently located in the upper lobes, and ≤4 cm in diameter, nodules are often discovered on a routine chest radiograph in an asymptomatic patient. Calcification is uncommon. Coccidioidal pulmonary nodules can be difficult to distinguish radiographically from pulmonary malignancies. Like malignancies, coccidioidal nodules often enhance on positron emission tomography. However, routine CT often demonstrates multiple nodules in coccidioidomycosis. Biopsy is often required to distinguish between these two conditions.
Pulmonary cavities occur when a nodule extrudes its contents into the bronchus, resulting in a thin-walled shell. These cavities can be associated with persistent cough, hemoptysis, and pleuritic chest pain. Rarely, a cavity may rupture into the pleural space, causing pyopneumothorax. In such cases, patients present with acute dyspnea, and the chest radiograph reveals a collapsed lung with a pleural air-fluid level. Chronic or persistent pulmonary coccidioidomycosis manifests with prolonged symptoms of fever, cough, and weight loss and is radiographically associated with pulmonary scarring, fibrosis, and cavities. It occurs most commonly in patients who already have chronic lung disease due to other etiologies.
In some cases, primary pneumonia presents as a diffuse reticulonodular pulmonary process (detected by plain chest radiography) in association with dyspnea and fever. Primary diffuse coccidioidal pneumonia may occur in settings of intense environmental exposure or profoundly suppressed cellular immunity (e.g., in patients with AIDS), with unrestrained fungal growth that is frequently associated with fungemia.
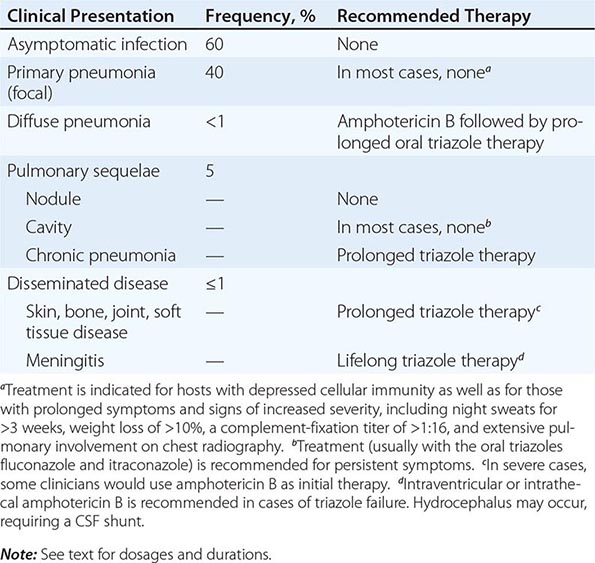
Clinical dissemination outside the thoracic cavity occurs in fewer than 1% of infected individuals. Dissemination is more likely to occur in male patients, particularly those of African-American or Filipino ancestry, and in persons with depressed cellular immunity, including patients with HIV infection and peripheral-blood CD4+ T cell counts of <250/μL; those receiving chronic glucocorticoid therapy; those with allogeneic solid-organ transplants; and those being treated with tumor necrosis factor α antagonists. Women who acquire infection during the second or third trimester of pregnancy also are at risk for disseminated disease. Common sites for dissemination include the skin, bone, joints, soft tissues, and meninges. Dissemination may follow symptomatic or asymptomatic pulmonary infection and may involve only one site or multiple anatomic foci. When it occurs, clinical dissemination is usually evident within the first few months after primary pulmonary infection.
Coccidioidal meningitis, if untreated, is uniformly fatal. Patients usually present with a persistent headache, which is sometimes accompanied by lethargy and confusion. Nuchal rigidity, if present, is not severe. Examination of cerebrospinal fluid (CSF) demonstrates lymphocytic pleocytosis with profound hypoglycorrhachia and elevated protein levels. CSF eosinophilia is occasionally documented. With or without appropriate therapy, patients may develop hydrocephalus, which presents clinically as a marked decline in mental status, often with gait disturbances.
DIAGNOSIS
As mentioned above, coccidioidomycosis is often misdiagnosed as community-acquired bacterial pneumonia. Clues that suggest a diagnosis of coccidioidomycosis include peripheral-blood eosinophilia, hilar or mediastinal adenopathy on radiographic imaging, marked fatigue, and failure to improve with antibiotic therapy.
Serology plays an important role in establishing a diagnosis of coccidioidomycosis. Several techniques are available, including the traditional tube-precipitin (TP) and complement-fixation (CF) assays, immunodiffusion (IDTP and IDCF), and enzyme immunoassay (EIA) to detect IgM and IgG antibodies. TP and IgM antibodies are found in serum soon after infection and persist for weeks. They are not useful for gauging disease progression and are not found in the CSF. The CF and IgG antibodies occur later in the course of the disease and persist longer than TP and IgM antibodies. Rising CF titers are associated with clinical progression, and the presence of CF antibody in CSF is indicative of coccidioidal meningitis. Antibodies disappear over time in persons whose clinical illness resolves.
Because of its commercial availability, the coccidioidal EIA is frequently used as a screening tool for coccidioidal serology. There has been concern that the IgM EIA is occasionally falsely positive, particularly in asymptomatic individuals. In addition, while the sensitivity and specificity of the IgG EIA appear to be higher than those of the CF and IDCF assays, the optical density obtained in the EIA does not correlate with the serologic titer of either of the latter tests.
Coccidioides grows within 3–7 days at 37°C on a variety of artificial media, including blood agar. Therefore, it is always useful to obtain samples of sputum or other respiratory fluids and tissues for culture in suspected cases of coccidioidomycosis. The clinical laboratory should be alerted to the possibility of this diagnosis, since Coccidioides poses a significant laboratory hazard if it is inadvertently inhaled. The organism can also be identified directly. While treatment of samples with potassium hydroxide is rarely fruitful in establishing the diagnosis, examination of sputum or other respiratory fluids after Papanicolaou or Gomori methenamine silver staining reveals spherules in a significant proportion of patients with pulmonary coccidioidomycosis. For fixed tissues (e.g., those obtained from biopsy specimens), spherules with surrounding inflammation can be demonstrated with hematoxylin-eosin or Gomori methenamine silver staining.
A commercially available test for coccidioidal antigenuria and antigenemia has been developed and appears to be particularly useful in immunosuppressed patients with severe or disseminated disease. False-positive results may occur in cases of histoplasmosis or blastomycosis. Some laboratories offer genomic detection by polymerase chain reaction.
PREVENTION
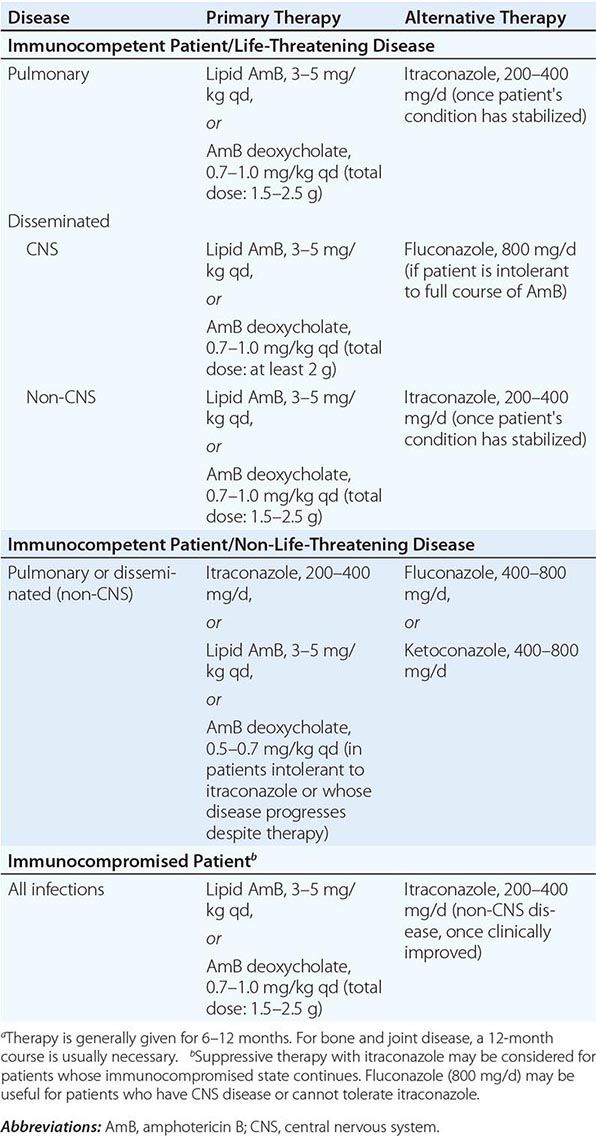
There are no proven methods to reduce the risk of acquiring coccidioidomycosis among residents of an endemic region, but avoidance of direct contact with uncultivated soil or with visible dust containing soil is reasonable. For individuals with suppressed cellular immunity, the risk of developing symptomatic coccidioidomycosis is greater than that in the general population. Among those about to undergo allogeneic solid-organ transplantation, antifungal therapy is appropriate when there is evidence of active or recent coccidioidomycosis. Several cases of donor-transmitted coccidioidomycosis have occurred during transplantation. If possible, donors from an endemic region should be screened for coccidioidomycosis before transplantation. Data on the use of antifungal agents for prophylaxis in other situations are limited. The administration of an antifungal drug to prevent symptomatic coccidioidomycosis is not recommended for HIV-1-infected patients who live in an endemic region. Most experts would administer a triazole to patients with a history of active coccidioidomycosis or a positive coccidioidal serology in whom therapy with tumor necrosis factor α antagonists is being initiated.
238 | Blastomycosis |
Blastomycosis is a systemic pyogranulomatous infection, involving primarily the lungs, that follows inhalation of the conidia of Blastomyces dermatitidis. Pulmonary blastomycosis varies from an asymptomatic infection to acute or chronic pneumonia. Hematogenous dissemination to skin, bones, and the genitourinary system is common; however, almost any organ can be involved.
ETIOLOGIC AGENT
B. dermatitidis is the asexual state of Ajellomyces dermatitidis. Two serotypes have been identified on the basis of the presence or absence of the A antigen. Distinct genotypic groups have been differentiated by rDNA polymerase chain reaction restriction fragment length polymorphisms and microsatellite markers. B. dermatitidis exhibits thermal dimorphism, growing as the mycelial phase at room temperature and as the yeast phase at 37°C. Primary isolation in the laboratory is most dependable for the mycelial phase incubated at 30°C. Definitive identification usually requires conversion to the yeast phase at 37°C or—now more commonly—the use of nucleic acid amplification techniques that detect mycelial-phase growth. Under the microscope, the yeast cells are usually 8–15 μm in diameter, have thick refractile cell walls, are multinucleate, and exhibit a single, large, broad-based bud (Fig. 238-1).
FIGURE 238-1 Blastomyces dermatitidis broad-based budding yeast in the aspirate of a chest wall abscess. Note the presence of multiple nuclei, the thickened cell wall, and the broad-based bud.
EPIDEMIOLOGY
Most cases of blastomycosis have been reported in North America. Endemic areas include the southeastern and south-central states bordering the Mississippi and Ohio river basins, the midwestern states, and the Canadian provinces bordering the Great Lakes. A small endemic area exists in New York and Canada along the St. Lawrence River. Acute blastomycosis is typically found only in North America, and the clinical presentation of blastomycosis in nonendemic areas is as a chronic disease.
![]() Outside North America, blastomycosis occurs sporadically in Nigeria, Zimbabwe, Tunisia, Saudi Arabia, Israel, Lebanon, and India. The disease has been reported most frequently in Africa.
Outside North America, blastomycosis occurs sporadically in Nigeria, Zimbabwe, Tunisia, Saudi Arabia, Israel, Lebanon, and India. The disease has been reported most frequently in Africa.
Early studies indicated that middle-aged men with outdoor occupations were at greatest risk. Reported outbreaks, however, do not suggest a predilection according to sex, age, race, occupation, or season. The specific niche in nature in which the organism resides remains uncertain; B. dermatitidis probably grows as microfoci in the warm, moist soil of wooded areas rich in organic debris. Inhalation of conidia following exposure to soil, whether related to work or recreation, appears to be the common factor associated with infection. Outbreaks of human disease may be preceded by the occurrence of disease in simultaneously exposed dogs. Zoonotic transmission is rare but has been reported in association with dog bites, pet kinkajou bites, cat scratches, and animal necropsies.
PATHOGENESIS
Alveolar macrophages and polymorphonuclear leukocytes are critical for phagocytosis and killing of the inhaled conidia of B. dermatitidis. The interaction of these mediators of the innate immune response with local host factors, such as lung surfactant, plays a significant role in inhibiting conversion to the pathogenic yeast form. This inhibition prevents the establishment of symptomatic disease and may account for the high frequency of asymptomatic infections in outbreaks. Once conversion to the thick-walled yeast form has occurred, phagocytosis and killing are much more difficult, and the development of clinically apparent infection is much more likely. Ultimately, the T lymphocyte response—specifically, a TH1 response—is the primary factor in limiting infection and dissemination. Moreover, yeast-phase conversion results in the expression of yeast phase–specific proteins such as the 120-kDa glycoprotein adhesin BAD-1 and the Blastomyces yeast phase–specific protein 1 (BYS1). BAD-1 has been well characterized as a virulence factor and is the major epitope for humoral and cellular immunity. The role of BYS1, putatively identified as a signal peptide, has not been determined.
CLINICAL MANIFESTATIONS
Acute pulmonary infection is often diagnosed in association with point-source outbreaks. Typical symptoms include the abrupt onset of fever, chills, pleuritic chest pain, arthralgias, and myalgias. Cough is initially nonproductive but frequently becomes purulent as disease progresses. Chest radiographs usually reveal alveolar infiltrates with consolidation. Pleural effusions and hilar adenopathy are uncommon. Most patients diagnosed with pulmonary blastomycosis have chronic indolent pneumonia with signs and symptoms of fever, weight loss, productive cough, and hemoptysis. The most common radiologic findings are alveolar infiltrates with or without cavitation, mass lesions that mimic bronchogenic carcinoma, and fibronodular infiltrates. Hematogenous dissemination to the skin, bones, and genitourinary tract occurs most often in association with chronic pulmonary disease. Although blastomycosis is not considered an opportunistic infection, immunosuppression has been recognized as a risk factor for more serious pulmonary involvement, including respiratory failure (adult respiratory distress syndrome) associated with miliary disease or diffuse pulmonary infiltrates. In the late stages of AIDS, mortality rates of ≥50% have been documented. Most deaths occur within the first few days of therapy. Solid-organ transplant recipients with endemic fungal infections, including both histoplasmosis and blastomycosis, frequently have more severe pulmonary disease as well as dissemination. Blastomycosis has been associated with a mortality rate of 36% in these patients.
![]() In Africa, pulmonary cases typically include bony involvement (frequently of the vertebrae), with subcutaneous abscesses of the chest wall or legs. All of the manifestations seen in African patients fall within the spectrum of blastomycosis observed in North America. The increased prevalence of chronic and disseminated bone disease in these patients may reflect a delay in diagnosis in regions where spinal disease is often treated empirically as tuberculosis.
In Africa, pulmonary cases typically include bony involvement (frequently of the vertebrae), with subcutaneous abscesses of the chest wall or legs. All of the manifestations seen in African patients fall within the spectrum of blastomycosis observed in North America. The increased prevalence of chronic and disseminated bone disease in these patients may reflect a delay in diagnosis in regions where spinal disease is often treated empirically as tuberculosis.
Skin disease is the most common extrapulmonary manifestation of blastomycosis. Two types of skin lesions occur: verrucous (more common) and ulcerative. Osteomyelitis occurs in as many as one-fourth of B. dermatitidis infections. The vertebrae, pelvis, sacrum, skull, ribs, and long bones are most frequently involved. Patients with B. dermatitidis osteomyelitis often present with contiguous soft-tissue abscesses or chronic draining sinuses. In men, blastomycosis may involve the prostate and epididymis. Central nervous system (CNS) disease occurs in fewer than 5% of immunocompetent patients with blastomycosis. A recent multicenter review identified 22 patients with CNS disease, of whom 12 (54%) met at least one criterion for immunosuppression; although most cases of CNS blastomycosis are associated with infection at other sites, 22.7% of the reviewed cases had only CNS involvement. CNS disease, usually presenting as a brain abscess, has been reported in ~40% of cases in patients with AIDS. Less common forms of CNS disease are cranial or spinal epidural abscess and meningitis.
DIAGNOSIS
Definitive diagnosis of blastomycosis requires growth of the organism from sputum, bronchial washings, pus, or biopsy material. Specimens should be inoculated onto a fungal medium such as Sabouraud dextrose agar, with or without chloramphenicol. B. dermatitidis is generally visible in 5–10 days but may require incubation for up to 30 days if only a few organisms are present in the specimen. A presumptive diagnosis may be based on demonstration of the characteristic broad-based budding yeast by microscopic examination of wet preps of sputum in pneumonia or of skin-lesion scrapings. Serologic testing for antibodies to B. dermatitidis by complement fixation, immunodiffusion, or enzyme immunoassay is of little value for diagnosis because of limited sensitivity and specificity as well as cross-reactivity with other fungal antigens.
A Blastomyces antigen assay that detects antigen in urine and serum is commercially available and is reasonably sensitive and specific (MiraVista Diagnostics, Indianapolis, IN). Antigen detection appears to be more sensitive in urine than in serum. This antigen test may be useful for monitoring of patients during therapy or for early detection of relapse. Chemiluminescent DNA probes (AccuProbe; GenProbe Inc., San Diego, CA) are commonly used to confirm identification of B. dermatitidis once growth has been detected in culture. Repetitive sequence–based PCR is available (DiversiLab System; bioMérieux, Durham, NC). Molecular identification techniques are currently used only to supplement traditional diagnostic methods.
PROGNOSIS
Cure rates are 90–95% among compliant immunocompetent patients given itraconazole for mild to moderate pulmonary and extrapulmonary disease without CNS involvement. Bone and joint disease usually requires 12 months of therapy. The fewer than 5% of infections that relapse after an initial course of itraconazole usually respond well to a second treatment course.
ACKNOWLEDGMENT
The authors thank Dr. Stanley W. Chapman, Professor Emeritus, University of Mississippi, for his continued help and support and for his contributions to this chapter in an earlier edition.
239 | Cryptococcosis |
DEFINITION AND ETIOLOGY
Cryptococcus, a genus of yeast-like fungi, is the etiologic agent of cryptococcosis. Both species, C. neoformans and C. gattii, can cause cryptococcosis in humans. The two varieties of C. neoformans—grubii and neoformans—correlate with serotypes A and D, respectively. C. gattii, although not divided into varieties, also is antigenically diverse, encompassing serotypes B and C. Most clinical microbiology laboratories do not routinely distinguish between C. neoformans and C. gattii, or among varieties, but rather identify and report all isolates simply as C. neoformans.
EPIDEMIOLOGY
Cryptococcosis was first described in the 1890s but remained relatively rare until the mid-twentieth century, when advances in diagnosis and increases in the number of immunosuppressed individuals markedly raised its reported prevalence. Although serologic evidence of cryptococcal infection is common among immunocompetent individuals, cryptococcal disease (cryptococcosis) is relatively rare in the absence of impaired immunity. Individuals at high risk for disease due to C. neoformans include patients with hematologic malignancies, recipients of solid organ transplants who require ongoing immunosuppressive therapy, persons whose medical conditions necessitate glucocorticoid therapy, and patients with advanced HIV infection and CD4+ T lymphocyte counts of <200/μL. In contrast, C. gattii–related disease is not associated with specific immune deficits and often occurs in immunocompetent individuals.
![]() Cryptococcal infection is acquired from the environment. C. neoformans and C. gattii inhabit different ecologic niches. C. neoformans is frequently found in soils contaminated with avian excreta and can easily be recovered from shaded and humid soils contaminated with pigeon droppings. In contrast, C. gattii is not found in bird feces. Instead, it inhabits a variety of arboreal species, including several types of eucalyptus tree. C. neoformans strains are found throughout the world; however, var. grubii (serotype A) strains are far more common than var. neoformans (serotype D) strains among both clinical and environmental isolates. The geographic distribution of C. gattii was thought to be largely limited to tropical regions until an outbreak of cryptococcosis caused by a new serotype B strain began in Vancouver in 1999. This outbreak has extended into the United States, and C. gattii infections are being encountered increasingly in several states in the Pacific Northwest.
Cryptococcal infection is acquired from the environment. C. neoformans and C. gattii inhabit different ecologic niches. C. neoformans is frequently found in soils contaminated with avian excreta and can easily be recovered from shaded and humid soils contaminated with pigeon droppings. In contrast, C. gattii is not found in bird feces. Instead, it inhabits a variety of arboreal species, including several types of eucalyptus tree. C. neoformans strains are found throughout the world; however, var. grubii (serotype A) strains are far more common than var. neoformans (serotype D) strains among both clinical and environmental isolates. The geographic distribution of C. gattii was thought to be largely limited to tropical regions until an outbreak of cryptococcosis caused by a new serotype B strain began in Vancouver in 1999. This outbreak has extended into the United States, and C. gattii infections are being encountered increasingly in several states in the Pacific Northwest.
The global burden of cryptococcosis was recently estimated at ~1 million cases, with >600,000 deaths annually. Thus cryptococci are important human pathogens. Since the onset of the HIV pandemic in the early 1980s, the overwhelming majority of cryptococcosis cases have occurred in patients with AIDS (Chap. 226). To comprehend the impact of HIV infection on the epidemiology of cryptococcosis, it is instructive to note that in the early 1990s there were >1000 cases of cryptococcal meningitis each year in New York City—a figure far exceeding that for all cases of bacterial meningitis. With the advent of effective antiretroviral therapy, the incidence of AIDS-related cryptococcosis has been sharply reduced among treated individuals. Thus most cases of cryptococcosis now occur in resource-limited regions of the world. The disease remains distressingly common in regions where antiretroviral therapy is not readily available (e.g., parts of Africa and Asia); in these regions, up to one-third of patients with AIDS have cryptococcosis. Among HIV-infected persons, those with a decreased percentage of memory B cells expressing IgM may be at greater risk for cryptococcosis.
PATHOGENESIS
Cryptococcal infection is acquired by inhalation of aerosolized infectious particles. The exact nature of these particles is not known; the two leading candidate forms are small desiccated yeast cells and basidiospores. Little is known about the pathogenesis of initial infection. Serologic studies have shown that cryptococcal infection is acquired in childhood, but it is not known whether the initial infection is symptomatic. Given that cryptococcal infection is common while disease is rare, the consensus is that pulmonary defense mechanisms in immunologically intact individuals are highly effective at containing this fungus. It is not clear whether initial infection leads to a state of immunity or whether most individuals are subject throughout life to frequent and recurrent infections that resolve without clinical disease. However, evidence indicates that some human cryptococcal infections lead to a state of latency in which viable organisms are harbored for prolonged periods, possibly in granulomas. Thus the inhalation of cryptococcal cells and/or spores can be followed by either clearance or establishment of the latent state. The consequences of prolonged harboring of cryptococcal cells in the lung are not known, but evidence from animal studies indicates that the organisms’ prolonged presence could alter the immunologic milieu in the lung and predispose to allergic airway disease.
Cryptococcosis usually presents clinically as chronic meningoencephalitis. The mechanisms by which the fungus undergoes extrapulmonary dissemination and enters the central nervous system (CNS) remain poorly understood. The mechanism by which cryptococcal cells cross the blood–brain barrier is a subject of intensive study. Current evidence suggests that both direct fungal-cell migration across the endothelium and fungal-cell carriage inside macrophages as “Trojan horse” invaders can occur. Cryptococcus species have well-defined virulence factors that include the expression of the polysaccharide capsule, the ability to make melanin, and the elaboration of enzymes (e.g., phospholipase and urease) that enhance the survival of fungal cells in tissue. Among these virulence factors, the capsule and melanin production have been most extensively studied. The cryptococcal capsule is antiphagocytic, and the capsular polysaccharide has been associated with numerous deleterious effects on host immune function. Cryptococcal infections can elicit little or no tissue inflammatory response. The immune dysfunction seen in cryptococcosis has been attributed to the release of copious amounts of capsular polysaccharide into tissues, where it probably interferes with local immune responses (Fig. 239-1). In clinical practice, the capsular polysaccharide is the antigen that is measured as a diagnostic marker of cryptococcal infection.
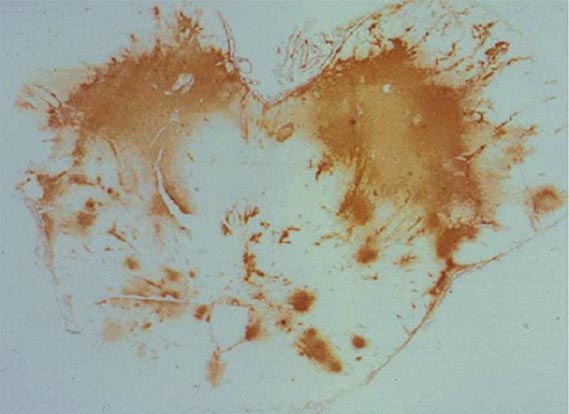
FIGURE 239-1 Cryptococcal antigen in human brain tissue, as revealed by immunohistochemical staining. Brown areas show polysaccharide deposits in the midbrain of a patient who died of cryptococcal meningitis. (Reprinted with permission from SC Lee et al: Hum Pathol 27:839, 1996.)
CLINICAL MANIFESTATIONS
The clinical manifestations of cryptococcosis reflect the site of fungal infection. The spectrum of disease caused by Cryptococcus species consists predominantly of meningoencephalitis and pneumonia, but skin and soft tissue infections also occur; in fact, cryptococcosis can affect any tissue or organ. CNS involvement usually presents as signs and symptoms of chronic meningitis, such as headache, fever, lethargy, sensory deficits, memory deficits, cranial nerve paresis, vision deficits, and meningismus. Cryptococcal meningitis differs from bacterial meningitis in that many Cryptococcus-infected patients present with symptoms of several weeks’ duration. In addition, classic characteristics of meningeal irritation, such as meningismus, may be absent in cryptococcal meningitis. Indolent cases can present as subacute dementia. Meningeal cryptococcosis can lead to sudden catastrophic vision loss.
Pulmonary cryptococcosis usually presents as cough, increased sputum production, and chest pain. Patients infected with C. gattii can present with granulomatous pulmonary masses known as cryptococcomas. Fever develops in a minority of cases. Like CNS disease, pulmonary cryptococcosis can follow an indolent course, and the majority of cases probably do not come to clinical attention. In fact, many cases are discovered incidentally during the workup of an abnormal chest radiograph obtained for other diagnostic purposes. Pulmonary cryptococcosis can be associated with antecedent diseases such as malignancy, diabetes, and tuberculosis.
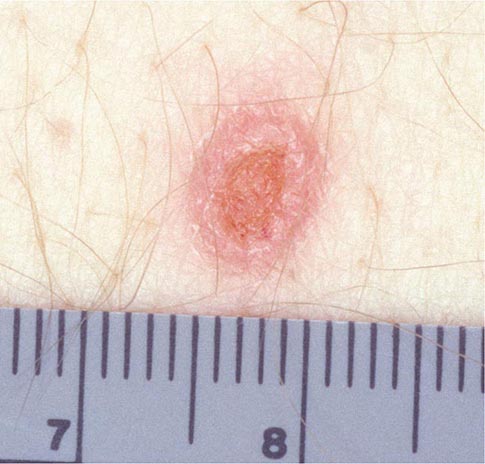
Skin lesions are common in patients with disseminated cryptococcosis and can be highly variable, including papules, plaques, purpura, vesicles, tumor-like lesions, and rashes. The spectrum of cryptococcosis in HIV-infected patients is so varied and has changed so much since the advent of antiretroviral therapy that a distinction between HIV-related and HIV-unrelated cryptococcosis is no longer pertinent. In patients with AIDS and solid organ transplant recipients, the lesions of cutaneous cryptococcosis often resemble those of molluscum contagiosum (Fig. 239-2; Chap. 220e).
FIGURE 239-2 Disseminated fungal infection. A liver transplant recipient developed six cutaneous lesions similar to the one shown. Biopsy and serum antigen testing demonstrated Cryptococcus. Important features of the lesion include a benign-appearing fleshy papule with central umbilication resembling molluscum contagiosum. (Photo courtesy of Dr. Lindsey Baden; with permission.)
DIAGNOSIS
A diagnosis of cryptococcosis requires the demonstration of yeast cells in normally sterile tissues. Visualization of the capsule of fungal cells in cerebrospinal fluid (CSF) mixed with India ink is a useful rapid diagnostic technique. Cryptococcal cells in India ink have a distinctive appearance because their capsules exclude ink particles. However, the CSF India ink examination may yield negative results in patients with a low fungal burden. This examination should be performed by a trained individual, since leukocytes and fat globules can sometimes be mistaken for fungal cells. Cultures of CSF and blood that are positive for cryptococcal cells are diagnostic for cryptococcosis. In cryptococcal meningitis, CSF examination usually reveals evidence of chronic meningitis with mononuclear cell pleocytosis and increased protein levels. A particularly useful test is cryptococcal antigen (CRAg) detection in CSF and blood. The assay is based on serologic detection of cryptococcal polysaccharide and is both sensitive and specific. A positive CRAg test provides strong presumptive evidence for cryptococcosis; however, because the result is often negative in pulmonary cryptococcosis, the test is less useful in the diagnosis of pulmonary disease and is of only limited usefulness in monitoring the response to therapy.
![]() In areas of Africa where there is a high prevalence of HIV infection, routine screening of blood for CRAg in HIV-infected patients with low CD4+ T lymphocyte counts may identify individuals at high risk of cryptococcal disease who are candidates for antifungal therapy. Similarly, CRAg screening has shown that a significant proportion of HIV-infected patients hospitalized with pneumonia in Thailand harbor cryptococcal infection. Inexpensive point-of-care CRAg tests that are under development could be of great diagnostic benefit in resource-limited regions.
In areas of Africa where there is a high prevalence of HIV infection, routine screening of blood for CRAg in HIV-infected patients with low CD4+ T lymphocyte counts may identify individuals at high risk of cryptococcal disease who are candidates for antifungal therapy. Similarly, CRAg screening has shown that a significant proportion of HIV-infected patients hospitalized with pneumonia in Thailand harbor cryptococcal infection. Inexpensive point-of-care CRAg tests that are under development could be of great diagnostic benefit in resource-limited regions.
PROGNOSIS AND COMPLICATIONS
Even with antifungal therapy, cryptococcosis is associated with high rates of morbidity and death. For the majority of patients with cryptococcosis, the most important prognostic factors are the extent and the duration of the underlying immunologic deficits that predisposed them to develop the disease. Therefore, cryptococcosis is often curable with antifungal therapy in individuals with no apparent immunologic dysfunction, but, in patients with severe immunosuppression (e.g., those with AIDS), the best that can be hoped for is that antifungal therapy will induce remission, which can then be maintained with lifelong suppressive therapy. Before the advent of antiretroviral therapy, the median overall survival period for AIDS patients with cryptococcosis was <1 year. Cryptococcosis in patients with underlying neoplastic disease has a particularly poor prognosis. For CNS cryptococcosis, poor prognostic markers are a CSF assay positive for yeast cells on initial India ink examination (evidence of a heavy fungal burden), high CSF pressure, low CSF glucose levels, low CSF pleocytosis (<2/μL), recovery of yeast cells from extraneural sites, absence of antibody to capsular polysaccharide, a CSF or serum cryptococcal antigen level of ≥1:32, and concomitant glucocorticoid therapy or hematologic malignancy. A response to treatment does not guarantee cure since relapse of cryptococcosis is common even among patients with relatively intact immune systems. Complications of CNS cryptococcosis include cranial nerve deficits, vision loss, and cognitive impairment.
PREVENTION
No vaccine is available for cryptococcosis. In patients at high risk (e.g., those with advanced HIV infection and CD4+ T lymphocyte counts of <200/μL), primary prophylaxis with fluconazole (200 mg/d) is effective in reducing the prevalence of disease. Since antiretroviral therapy raises the CD4+ T lymphocyte count, it constitutes an immunologic form of prophylaxis. However, cryptococcosis in the setting of immune reconstitution has been reported in patients with HIV infection and in recipients of solid organ transplants.
240 | Candidiasis |
The genus Candida encompasses more than 150 species, only a few of which cause disease in humans. With rare exceptions (although the exceptions are increasing in number), the human pathogens are C. albicans, C. guilliermondii, C. krusei, C. parapsilosis, C. tropicalis, C. kefyr, C. lusitaniae, C. dubliniensis, and C. glabrata. Ubiquitous in nature, they inhabit the gastrointestinal tract (including the mouth and oropharynx), the female genital tract, and the skin. Although cases of candidiasis have been described since antiquity in debilitated patients, the advent of Candida species as common human pathogens dates to the introduction of modern therapeutic approaches that suppress normal host defense mechanisms. Of these relatively recent advances, the most important is the use of antibacterial agents that alter the normal human microbiota and allow nonbacterial species to become more prevalent in the commensal flora. With the introduction of antifungal agents, the causes of Candida infections shifted from an almost complete dominance of C. albicans to the common involvement of C. glabrata and the other species listed above. The non-albicans species now account for approximately half of all cases of candidemia and hematogenously disseminated candidiasis. Recognition of this change is clinically important, since the various species differ in susceptibility to the newer antifungal agents. In developed countries, where medical therapeutics are commonly used, Candida species are now among the most common nosocomial pathogens.
Candida is a small, thin-walled, ovoid yeast that measures 4–6 μm in diameter and reproduces by budding. Organisms of this genus occur in three forms in tissue: blastospores, pseudohyphae, and hyphae. Candida grows readily on simple medium; lysis centrifugation enhances its recovery from blood. Species are identified by biochemical testing (currently with automated devices) or on special agar (e.g., CHROMagar).
EPIDEMIOLOGY
![]() Candida organisms are ubiquitous in nature; worldwide, these fungi are present in humans as commensals, in animals, in foods, and on inanimate objects. In developed countries, where advanced medical therapeutics are commonly used (see “Treatment,” below), Candida species are now among the most common health care–associated pathogens. In the United States, these species are the fourth most common isolates from the blood of hospitalized patients. In countries where advanced medical care is rarely available, mucocutaneous Candida infections, such as thrush, are more common than deep organ infections, which rarely occur; however, the incidence of deep organ candidiasis increases steadily as advances in health care—such as therapy with broad-spectrum antibiotics, more aggressive treatment of cancer, and the use of immunosuppression for sustaining organ transplants—are introduced and implemented. In recent decades, as a result of the HIV epidemic, the incidence of thrush and Candida esophagitis has increased substantially. In aggregate, the global incidence of infections due to Candida species has risen steadily over the past few decades.
Candida organisms are ubiquitous in nature; worldwide, these fungi are present in humans as commensals, in animals, in foods, and on inanimate objects. In developed countries, where advanced medical therapeutics are commonly used (see “Treatment,” below), Candida species are now among the most common health care–associated pathogens. In the United States, these species are the fourth most common isolates from the blood of hospitalized patients. In countries where advanced medical care is rarely available, mucocutaneous Candida infections, such as thrush, are more common than deep organ infections, which rarely occur; however, the incidence of deep organ candidiasis increases steadily as advances in health care—such as therapy with broad-spectrum antibiotics, more aggressive treatment of cancer, and the use of immunosuppression for sustaining organ transplants—are introduced and implemented. In recent decades, as a result of the HIV epidemic, the incidence of thrush and Candida esophagitis has increased substantially. In aggregate, the global incidence of infections due to Candida species has risen steadily over the past few decades.
PATHOGENESIS
In the most serious form of Candida infection, the organisms disseminate hematogenously and form microabscesses and small macroabscesses in major organs. Although the exact mechanism is not known, Candida probably enters the bloodstream from mucosal surfaces after growing to large numbers as a consequence of bacterial suppression by antibacterial drugs; alternatively, in some instances, the organism may enter from the skin. A change from the blastospore stage to the pseudohyphal and hyphal stages is generally considered integral to the organism’s penetration into tissue. However, C. glabrata can cause extensive infection even though it does not transform into pseudohyphae or hyphae. Adherence to both epithelial and endothelial cells, thought to be the first step in invasion and infection, has been studied extensively, and several adhesins have been identified. Biofilm formation also is considered important in pathogenesis. Numerous reviews of cases of hematogenously disseminated candidiasis have identified the predisposing factors or conditions associated with disseminated disease (Table 240-1). Women who receive antibacterial agents may develop vaginal candidiasis.
WELL-RECOGNIZED FACTORS AND CONDITIONS PREDISPOSING TO HEMATOGENOUSLY DISSEMINATED CANDIDIASIS |
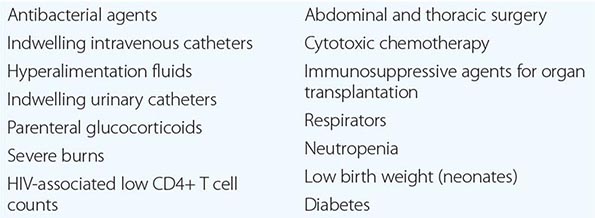
Innate immunity is the most important defense mechanism against hematogenously disseminated candidiasis, and the neutrophil is the most important component of this defense. Macrophages also play an important defensive role. STAT1, Dectin-1, CARD9, and TH1 and TH17 lymphocytes contribute significantly to innate defense (see “Clinical Manifestations,” below). Although many immunocompetent individuals have antibodies to Candida, the role of these antibodies in defense against the organism is not clear. Multiple genetic polymorphisms that predispose to disseminated candidiasis will most likely be identified in future studies.
CLINICAL MANIFESTATIONS
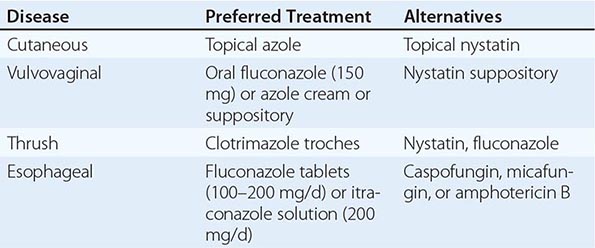
Mucocutaneous Candidiasis Thrush is characterized by white, adherent, painless, discrete or confluent patches in the mouth, on the tongue, or in the esophagus, occasionally with fissuring at the corners of the mouth. This form of disease caused by Candida can also occur at points of contact with dentures. Organisms are identifiable in gram-stained scrapings from lesions. The occurrence of thrush in a young, otherwise healthy-appearing person should prompt an investigation for underlying HIV infection. More commonly, thrush is seen as a nonspecific manifestation of severe debilitating illness. Vulvovaginal candidiasis is accompanied by pruritus, pain, and vaginal discharge which is usually thin but may contain whitish “curds” in severe cases. A subset of patients with recurrent vulvovaginitis have a deficiency in the surface expression of Dectin-1, a major recognition factor for β-glucan on Candida. This deficiency leads to suboptimal functioning of the CARD9 pathway, which ultimately increases the propensity for recurrent vaginal infections.
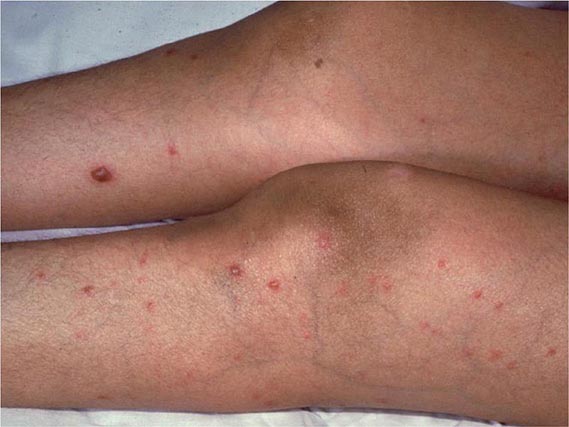
Other Candida skin infections include paronychia, a painful swelling at the nail-skin interface; onychomycosis, a fungal nail infection rarely caused by this genus; intertrigo, an erythematous irritation with redness and pustules in the skin folds; balanitis, an erythematous-pustular infection of the glans penis; erosio interdigitalis blastomycetica, an infection between the digits of the hands or toes; folliculitis, with pustules developing most frequently in the area of the beard; perianal candidiasis, a pruritic, erythematous, pustular infection surrounding the anus; and diaper rash, a common erythematous-pustular perineal infection in infants. Generalized disseminated cutaneous candidiasis, another form of infection that occurs primarily in infants, is characterized by widespread eruptions over the trunk, thorax, and extremities. The diagnostic macronodular lesions of hematogenously disseminated candidiasis (Fig. 240-1) indicate a high probability of dissemination to multiple organs as well as the skin. While the lesions are seen predominantly in immunocompromised patients treated with cytotoxic drugs, they may also develop in patients without neutropenia.
FIGURE 240-1 Macronodular skin lesions associated with hematogenously disseminated candidiasis. Candida organisms are usually but not always visible on histopathologic examination. The fungi grow when a portion of the biopsied specimen is cultured. Therefore, for optimal identification, both histopathology and culture should be performed. (Image courtesy of Dr. Noah Craft and the Victor Newcomer collection at UCLA, archived by Logical Images, Inc.; with permission.)
Chronic mucocutaneous candidiasis is a heterogeneous infection of the hair, nails, skin, and mucous membranes that persists despite intermittent therapy. The onset of disease usually comes in infancy or within the first two decades of life but in rare cases comes in later life. The condition may be mild and limited to a specific area of the skin or nails, or it may take a severely disfiguring form (Candida granuloma) characterized by exophytic outgrowths on the skin. Chronic mucocutaneous candidiasis is usually associated with specific immunologic dysfunction; most frequently reported is a failure of T lymphocytes to proliferate or to excrete cytokines in response to stimulation by Candida antigens in vitro. A subset of the affected patients have mutations in the STAT1 gene resulting in an insufficiency of interferon γ, interleukin 17, and interleukin 22.
Approximately half of patients with chronic mucocutaneous candidiasis have associated endocrine abnormalities that together are designated the autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy (APECED) syndrome. This syndrome is due to mutations in the autoimmune regulator (AIRE) gene and is most prevalent among Finns, Iranian Jews, Sardinians, northern Italians, and Swedes. Conditions that usually follow the onset of the disease include hypoparathyroidism, adrenal insufficiency, autoimmune thyroiditis, Graves’ disease, chronic active hepatitis, alopecia, juvenile-onset pernicious anemia, malabsorption, and primary hypogonadism. In addition, dental enamel dysplasia, vitiligo, pitted nail dystrophy, and calcification of the tympanic membranes may occur. Patients with chronic mucocutaneous candidiasis rarely develop hematogenously disseminated candidiasis, probably because their neutrophil function remains intact.
Deeply Invasive Candidiasis Deeply invasive Candida infections may or may not be due to hematogenous seeding. Deep esophageal infection may result from penetration by organisms from superficial esophageal erosions; joint or deep wound infection from contiguous spread of organisms from the skin; kidney infection from catheter-initiated spread of organisms through the urinary tract; infection of intraabdominal organs and the peritoneum from perforation of the gastrointestinal tract; and gallbladder infection from retrograde migration of organisms from the gastrointestinal tract into the biliary drainage system.
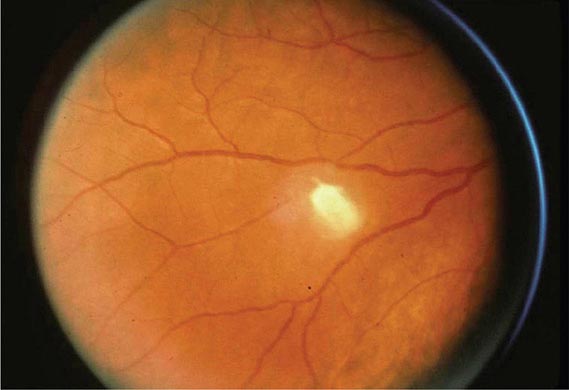
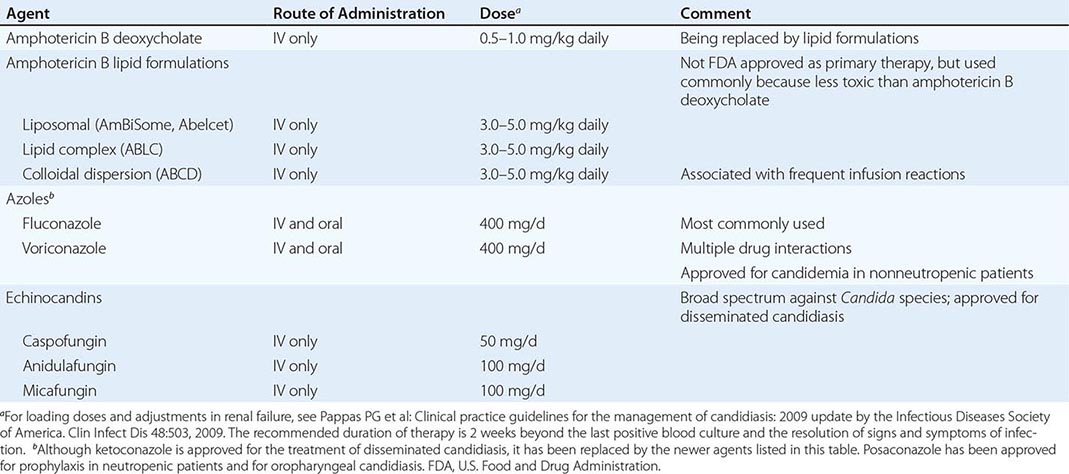
However, far more commonly, deeply invasive candidiasis results from hematogenous seeding of various organs as a complication of candidemia. Once the organism gains access to the intravascular compartment (either from the gastrointestinal tract or, less often, from the skin through the site of an indwelling intravascular catheter), it may spread hematogenously to a variety of deep organs. The brain, chorioretina (Fig. 240-2), heart, and kidneys are most commonly infected and the liver and spleen less commonly so (most often in neutropenic patients). In fact, nearly any organ can become involved, including the endocrine glands, pancreas, heart valves (native or prosthetic), skeletal muscle, joints (native or prosthetic), bone, and meninges. Candida organisms can also spread hematogenously to the skin and cause classic macronodular lesions (Fig. 240-1). Frequently, painful muscular involvement also is evident beneath the area of affected skin. Chorioretinal involvement and skin involvement are highly significant, since both findings are associated with a very high probability of abscess formation in multiple deep organs as a result of generalized hematogenous seeding. Ocular involvement (Fig. 240-2) may require specific treatment (e.g., partial vitrectomy or intraocular injection of antifungal agents) to prevent permanent blindness. An ocular examination is indicated for all patients with candidemia, whether or not they have ocular manifestations.
FIGURE 240-2 Hematogenous Candida endophthalmitis. A classic off-white lesion projecting from the chorioretina into the vitreous causes the surrounding haze. The lesion is composed primarily of inflammatory cells rather than organisms. Lesions of this type may progress to cause extensive vitreal inflammation and eventual loss of the eye. Partial vitrectomy, combined with IV and possibly intravitreal antifungal therapy, may be helpful in controlling the lesions. (Image courtesy of Dr. Gary Holland; with permission.)
DIAGNOSIS
The diagnosis of Candida infection is established by visualization of pseudohyphae or hyphae on wet mount (saline and 10% KOH), tissue Gram’s stain, periodic acid–Schiff stain, or methenamine silver stain in the presence of inflammation. Absence of organisms on hematoxylin-eosin staining does not reliably exclude Candida infection. The most challenging aspect of diagnosis is determining which patients with Candida isolates have hematogenously disseminated candidiasis. For instance, recovery of Candida from sputum, urine, or peritoneal catheters may indicate mere colonization rather than deep-seated infection, and Candida isolation from the blood of patients with indwelling intravascular catheters may reflect inconsequential seeding of the blood from or growth of the organisms on the catheter. Despite extensive research into both antigen and antibody detection systems, there is currently no widely available and validated diagnostic test to distinguish patients with inconsequential seeding of the blood from those whose positive blood cultures represent hematogenous dissemination to multiple organs. Many studies are under way to establish the utility of the β-glucan test; at present, its greatest utility is its negative predictive value (∼90%). Meanwhile, the presence of ocular or macronodular skin lesions is highly suggestive of widespread infection of multiple deep organs. Although extensive research is being conducted on other tests for infection, such as PCR, none of these tests is fully validated or widely available at present.