Learning Outcomes
After completing this chapter, you will be able to
 Define compounding.
Define compounding.
 Describe the basics of intravenous drug therapy.
Describe the basics of intravenous drug therapy.
 Describe the key elements of working in laminar airflow workbenches.
Describe the key elements of working in laminar airflow workbenches.
 List the common types of contamination that may occur when working in a laminar flow hood and describe how to minimize the risks of these types of contamination.
List the common types of contamination that may occur when working in a laminar flow hood and describe how to minimize the risks of these types of contamination.
 Perform basic manipulations needed to prepare a sterile product by using aseptic technique.
Perform basic manipulations needed to prepare a sterile product by using aseptic technique.
 Describe the risks of handling cytotoxic and hazardous drugs.
Describe the risks of handling cytotoxic and hazardous drugs.
 List the steps in drug preparation and handling that are unique to cytotoxic and hazardous drugs.
List the steps in drug preparation and handling that are unique to cytotoxic and hazardous drugs.
 List the typical ingredients of a total parenteral nutrition solution.
List the typical ingredients of a total parenteral nutrition solution.
 Describe the manual and automated means of preparing total parenteral nutrition solutions.
Describe the manual and automated means of preparing total parenteral nutrition solutions.
 Describe the benefits of having a formal intravenous admixture program.
Describe the benefits of having a formal intravenous admixture program.
 Describe how USP 797 has impacted the preparation of sterile products.
Describe how USP 797 has impacted the preparation of sterile products.
Key Terms
| aseptic technique | The technique and procedures designed to prevent contamination of drugs, packaging, equipment, or supplies by microorganisms during preparation. |
| biological safety cabinet | A vertical laminar airflow workbench (LAFW) used for the preparation of hazardous medications that confines airflow within the hood. |
| coring | Introducing particulate matter in the form of a plastic or rubber “core” or plug into a sterile fluid through the process of penetrating the outer seal of a vial or bag with a needle. |
Parenteral Drug Administration
Administration Systems for Parenteral Products
Aseptic Preparation of Parenteral Products
Preparation and Handling of Cytotoxic and Hazardous Drugs
Total Parenteral Nutrition Solutions
Pediatric Parenteral Drug Administration
|
Aseptic technique, along with its application to different dispensing and administration systems and dosage types, is the primary focus of this chapter. Potential risks of parenteral therapy are addressed in the next section in order to emphasize the importance of using proper techniques and appropriate caution when preparing these products. Nurses are often referred to as the primary caregivers involved in administering the products described in this chapter; however, in some hospitals or home care settings, the primary caregiver may be another health care professional, the patient, or the patient’s family members. The training and skill of the caregiver or caregivers should be taken into consideration when products are prepared and dispensed because this may influence the intravenous (IV) delivery system chosen for the patient.
The purpose of this chapter is to help the pharmacy technician develop a basic understanding of sterile products and the methods used to prepare them. This chapter should be mastered in sequence with the rest of the Manual for Pharmacy Technicians, especially chapters that have related information, including Chapter 4, Hospital Pharmacy Practice, Chapter 5, Home Care Pharmacy Practice, Chapter 13, Processing Medication Orders and Prescriptions, and Chapter 14, Pharmacy Calculations.
Many small hospitals prepare and administer hundreds of sterile products daily; larger hospitals may prepare thousands. As such, pharmacy technicians play an important role in the preparation of sterile products. In addition, many patients now receive intravenous drug therapy in the home. It is important that correct procedures are followed when preparing intravenous drug therapy to ensure that the final product is safe and effective. For example, a structured intravenous admixture program is needed to ensure the stability, sterility, and appropriate labeling of intravenous products. Technicians and pharmacists work as a team in the IV preparation environment, each with their own role and expertise, to ensure that the patient receives the right drug in the right amount, concentration, and dosage form.
Drugs are given parenterally if patients cannot take oral medications; if they have difficulty absorbing oral medications (i.e., absorptive capacity is limited); if a more rapid onset of action is desired (as in an emergency situation); or if the drug is not available in a suitable oral dosage form. Though the parenteral route of drug administration offers many advantages, it also has some unique preparation requirements to ensure that patients are not harmed.
Because the drug or solution is being injected directly into the body, it bypasses the body’s barriers to infection.1 Therefore, it is extremely important that the solution be sterile; that is, it must be free from bacteria or other living organisms. If a drug or solution that is contaminated with bacteria is inadvertently injected into a patient, the patient can suffer fatal adverse effects. Aseptic technique is the term used for all procedures and techniques utilized to keep a sterile product from becoming contaminated with live bacteria or other microorganisms.
Parenteral Drug Administration
Medications can be administered to patients in numerous ways. Medications not given to patients through the digestive tract (enterally) are referred to as parenterally administered. These can include intravenous (IV), intramuscular (IM), intrathecal (IT), epidural, intraarticular, intraarterial, intraocular, intraperitoneal, and subcutaneous (SQ, SC, SubQ) routes of administration. IV solutions are commonly administered to patients as a means of replacing body fluids and as a vehicle for introducing drugs into the body. Medications are not beneficial to the patient until they reach the blood and are distributed to the body. Medications given via IV are introduced directly into the blood and therefore have the most rapid onset of action. This has many benefits over oral medications, which have to be absorbed from the gastrointestinal tract, or IM medications, which have to be absorbed from the muscle mass. Intravenous medications are also beneficial in that they can be given to patients who are unconscious, uncooperative, nauseated, vomiting, or otherwise unable to take medications orally. Direct administration of IV medications into the blood also provides a predictable rate of administration. Some medications, however, are simply not suitable for IV administration due to their instability or adsorptive properties. Disadvantages of IV medications include the risk of infection, pain on injection, and immediate effect of the medication in the event of an error.
Special training is required for all personnel who prepare and administer sterile intravenous solutions. Basic aseptic technique should be used when handling parenteral dosage forms, as well as irrigations and ophthalmics (see Chapter 10, Medication Dosage Forms and Routes of Administration). Much of this chapter will address the intravenous route of administration because it is the most common route through which parenteral doses are administered in health systems today.
Risks of Intravenous Therapy
Intravenous therapy offers a rapid, direct means of administering many life-saving drugs and fluids. A high percentage of IV therapy is administered without any problems, but there are some risks. Many of the issues addressed in this training manual are aimed at teaching proper technique and therefore minimizing the potential for risks associated with these medications. What follows are some reported complications of IV therapy that may increase the risk to patients.3
Infection. Infections can result if a product contaminated with microorganisms or pathogens is infused into a patient. Because the IV route bypasses the body’s normal barrier system, microorganisms reach the bloodstream directly. These may be introduced into products during preparation, administration, production, or through improper storage. The rate of infection or sepsis due to a contaminated infusion has steadily decreased because health care practitioners and product manufacturers have implemented training, good manufacturing practices (GMPs), and quality assurance programs. Despite these efforts, human touch contamination (improper product handling) continues to be the most common source of IV-related contamination.
Air embolus. The incidence of an air embolus is low because many solutions are administered using infusion pumps equipped with an alarm that sounds when air is in the IV line. These are called air-in-line alarms. Solutions infused by gravity do not need alarms because the infusion automatically stops when there is no more fluid for gravity to push through the IV line. Even when a bag runs dry, large amounts of air are not infused. In adults, it takes 15–20 mL of air given quickly to result in harm, which may include shortness of breath, chest pain, blood pressure and heart rate changes, and even death. Infants and pediatric patients are adversely affected by much lower amounts of air.3 Air-eliminating filters are available on some IV sets, which also stop air bubbles and add another measure of safety.
Bleeding. Bleeding may or may not be caused by intravenous therapy. When the IV catheter is removed, bleeding may occur around the catheter site. Also, if the patient has a condition that results in prolonged bleeding time or takes an anticoagulant medication, extra care and caution should be used, especially when removing the catheter.
Allergic reaction. When a patient has an allergic reaction to a substance given parenterally, the reaction is usually more severe than if the same substance were given by another route (e.g., orally, topically, or rectally). One reason for this is that substances given parenterally cannot be retrieved like substances given by other routes. For example, substances administered topically can often be washed off, those given orally can be retrieved by inducing vomiting or by pumping the stomach, and those substances given rectally can be flushed out using an enema. When a drug that has a higher likelihood of causing allergic reactions (e.g., penicillin) is given intravenously, the patient should be monitored closely. If the likelihood of an allergic reaction is especially high and there is no alternative therapy allowing for risk mitigation, a test dose (a small amount of the drug that is often called a challenge) may be given to see how the patient reacts before administering the full dose of the medication.
Incompatibilities. Some drugs are incompatible with other drugs, containers, or solutions. If an incompatibility exists, the drug may precipitate, be inactivated, or adhere to the container. These outcomes are undesirable and may be difficult to detect with the naked eye. A visual inspection of the final product should always be performed to observe any cloudiness or signs of irregularity. Solutions with known or detectable incompatibilities should not be administered to patients.
Extravasation. Extravasation occurs when the IV catheter punctures and exits the vein under the skin, causing drugs to infuse or infiltrate into the tissue. Extravasation may happen when the catheter is being inserted or after it is in place if the extremity with the IV catheter is moved or flexed too much. Using a stiff arm board to prevent excessive movement near the catheter site may help maintain regular flow and prevent extravasation and infiltration. Extravasation and infiltration can be painful and usually requires that the IV be restarted in a different location. Some drugs, such as certain chemotherapy agents, may cause severe tissue damage if they infiltrate the tissue. Although there are medications that can alleviate some of the effects of the drug and hot or cold compresses can stop progression and reduce swelling, in some cases the tissue damage is severe enough to require surgery or can result in the loss of the limb.
Particulate Matter. Particulate matter refers to unwanted particles present in parenteral products. Particulate matter that is injected into the bloodstream can cause adverse effects to the patient. Some examples of particulate matter are glass fragments, hair, lint or cotton fibers, cardboard fragments, undissolved drug particles, and fragments of rubber stoppers, known as cores. Improvements in manufacturing processes have greatly reduced the presence of particulates in commercially available products. Similar care must be taken in the pharmacy so that particulate matter is not introduced into products.
 When using glass ampules, filters are required to prevent glass fragments from entering the compounded sterile product. All products should be visually inspected for particulate matter before dispensing.
When using glass ampules, filters are required to prevent glass fragments from entering the compounded sterile product. All products should be visually inspected for particulate matter before dispensing.
Some institutions may additionally use inline filters to help minimize the amount of particulate that reaches the patient, especially in situations where medications need to be prepared in emergency situations outside the controlled environment of a pharmacy.
Pyrogens. Pyrogens, the by-products or remnants of bacteria, can cause reactions (e.g., fever and chills) if injected in large enough amounts. Since a pyrogen can be present even after a solution has been sterilized, great care must be taken to ensure that these substances are not present in quantities that would harm the patient via filtration when appropriate. If the pyrogen is smaller than the filter being used, however, it may be introduced into the bloodstream.
Phlebitis. Phlebitis, or irritation of the vein, may be caused by the IV catheter, the drug itself due to its chemical properties or its concentration, the location of the IV site, the rate of administration, or the presence of particulate matter. The patient usually feels pain or discomfort along the path of the vein, which is often severe. Red streaking can also occur. If phlebitis is caused by a particular drug, it may be helpful to further dilute the drug, give it more slowly, or give it via an IV catheter placed in a larger vein with a higher, faster-moving volume of blood.
Types of IV Administration
Medications can be administered intravenously through several different systems or processes. While they are commonly larger volume solutions, they may not be in the case of pediatric doses. Infusions can be given either continuously or intermittently. While continuous infusions of IV fluids or medications are given over extended periods of time (often twenty-four hours or more), intermittent infusions of medications are given over a short period of time, such as over 15 minutes up to several hours. In most IV solutions, one or more drugs are added to the IV solution to prepare the final sterile product. The drug is referred to as the additive and the final product is referred to as the admixture.
 An IV injection is generally a small volume of solution administered directly from a syringe into the vein. When given over a short period of time, it is referred to as IV push. Solutions in IV bags or bottles that are administered over a longer period of time are known as IV infusions.
An IV injection is generally a small volume of solution administered directly from a syringe into the vein. When given over a short period of time, it is referred to as IV push. Solutions in IV bags or bottles that are administered over a longer period of time are known as IV infusions.
IV Containers
Large Volume Parenterals(LVPs). Large volume parenterals are defined as IV solutions greater than 100 mL in volume. LVPs are usually solutions of dilute dextrose and/or sodium chloride with or without drug additives. These are usually given as continuous infusions, but they may be used for intermittent infusions as well. These preparations may be used in their commercially available form or may have drug additives added in the pharmacy.
Small Volume Parenterals or “Piggyback”
Systems. A common method of preparing drugs is adding the drug solution to a small volume parenteral or piggyback (any IV solution less than or equal to 100 mL) and labeling it. The nurse simply attaches tubing to the piggyback and connects this secondary IV set to the primary IV set at the proximal Y-site (figure 16–1). Piggybacking offers the benefits of flexibility and ease of administration.
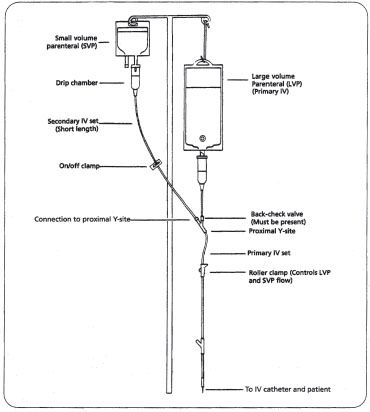
Figure 16–1. A small volume parenteral, or piggyback setup. Note that the piggyback hangs higher than the primary IV.
The piggyback is placed higher than the primary IV (usually an LVP) so that gravity causes the drug solution to run into the patient’s vein before the primary fluid. The back-check valve at the proximal Y-site closes while the piggyback is being administered, thus preventing the piggyback solution from entering the primary IV. Once the piggyback solution has infused, the primary IV resumes flowing. There are a number of systems that are variations of the basic piggyback concept.
Many drugs and doses for piggyback administration are available in premixed form. If premixed products are not stable for long periods of time at room temperature, they are often sold frozen, and thawed by the pharmacy shortly (hours or days) before being administered. Adding drugs to these solutions is generally not recommended, and most containers do not have an injection port. These solutions are administered and handled by the nurse in the same manner as other piggyback setups.
Figure 16–2. The ADD-Vantage® system setup is shown here. Note the special port at the top of the bag, which holds the medication vial.
Add-Vantage®. The Add-Vantage® system (figure 16–2) uses a specially designed bag and vial that contains drug for reconstitution. The vial is screwed into a special receptacle on the top of the bag. To reconstitute the drug, the vial’s stopper is removed by manipulations done on the outside of the bag and the stopper remains in the bag. The IV solution then flows from the bag into the vial and dissolves and/or dilutes the drug. The bag is inverted several times to mix the drug and the IV solution. The bag is then administered to the patient in a fashion similar to the traditional piggyback setup.
The act of screwing the vial onto the bag receptacle should be performed in a laminar airflow workbench (LAFW) unless meeting criteria of Immediate-Use preparations as defined by USP. The actual vial top and receptacle are sterile and shielded by a protective cover until used. The pharmacy technician removes the cover at the time the vial is screwed on.
The bag’s expiration date is usually thirty days after the date the vial is attached. The bag’s expiration date changes to the drug expiration date when the stopper is pulled and the drug is mixed, or activated. For this reason, the stopper is usually left intact by the pharmacy and is pulled by the nurse just prior to administering the dose. This way, changes in the medication order do not result in wasted doses.
Vial Spike Systems. The Add-a-Vial®, AddEase®, Vial Mate, and Mini-Bag Plus® systems are similar in concept to the Add-Vantage® system. The drug-containing vial is attached to the bag in the pharmacy but is not activated or mixed until just before administration. The Add-a-Vial® system uses a vial adapter. The adapter has a spike at each end; one is inserted into the drug vial and the other container, first remove the protective cap from the IV is inserted into the injection port of the bag. The MiniBag Plus® system (figure 16–3) uses a special container that has a vial adapter and a breakaway seal. The pharmacy is responsible for attaching the drug-containing vial to the bag.
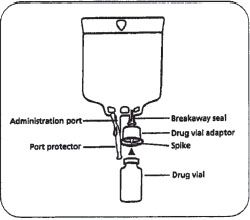
Figure 16–3. The Mini-Bag Plus® system has a special manufacturer’s bag equipped with a drug vial adapter. The adapter is pushed down on the vial and snapped into place. The Add-a-Vial® system operates on a similar principle except the drug vial adapter is separate from the bag and is spiked on both ends. It is attached to the drug vial first, then assembled with the bag. With both systems, the assembled product is sent to the nursing unit, where, just prior to administration, the breakaway seal is broken and the solution is mixed with the drug.
The Add-a-Vial® spike that is inserted into the bag is snapped off or the Mini-Bag Plus® breakaway seal is broken just before administration, allowing solution from the bag to enter the drug vial and be mixed. The system does not require special vials because the adapters are designed to fit commonly used vial sizes. Add-a-Vial® can be used with various manufacturers’ bags. MiniBag Plus® requires that the manufacturer’s bag be used because the drug vial adapter is attached. With each of these systems, it is important to ensure that the product is activated to ensure that the patient receives the dose of medication.
Flexible Plastic Bags. Flexible plastic bags made of polyvinyl chloride (PVC) are used frequently. They are easier to store, are less breakable than glass bottles, and eliminate the need to vent the container when removing fluid.
PVC bags are available in several sizes and contain a variety of solutions. They are packaged in plastic overwrap designed to limit fluid loss. The protective overwrap should not be removed from a PVC bag until it is ready to be used. To minimize air turbulence in the critical area, position the injection port of a PVC bag, which is covered by an outside tip diaphragm, toward the HEPA filter when preparing an IV admixture.
 Various PVC bags will require a revision of the expiration date once the overwrap is removed. Review the product package insert for proper dating of IV bags once removed from their overwrap.
Various PVC bags will require a revision of the expiration date once the overwrap is removed. Review the product package insert for proper dating of IV bags once removed from their overwrap.
To add a drug to a PVC bag, swab the injection port, then insert a needle into the injection port and inject the appropriate volume of drug fluid. Use a needle at least one inch long because the injection port of the PVC bag has two diaphragms that must be pierced (figure 16–4). The outside diaphragm is the outside latex tip; the inside diaphragm, which is plastic, is about 3/8 inch inside the injection portal. Note that individual manufacturer’s products may differ in appearance, but the design concept is the same.
Glass Containers. To add a drug to a glass infusion container, first remove the protective cap from the IV bottle. Swab the rubber stopper with alcohol, let it dry, and then inject the drug fluid. To insert needles through rubber stoppers, use a non-coring technique (figure 16–5). After injecting the drug into the IV bottle, remove the bottle vacuum by removing the syringe needle used to inject the drug fluid and prepare a new empty syringe. Reattach the needle to the new empty syringe and remove the plunger. Insert the needle with the syringe barrel into the bottle. After admixing, place a protective seal over the stopper of the glass container before removing it from the LAFW.
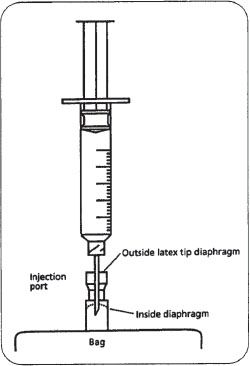
Figure 16–4. A syringe penetrating the injection port of a PVC bag. The needle must be long enough to penetrate the inside diaphragm. (Note: figure is not drawn to scale.)
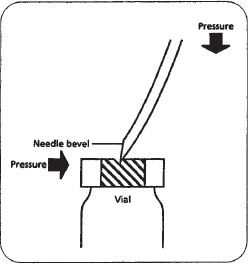
Figure 16–5. A non-coring technique of piercing a vial with a needle. Note that the needle is held on an angle — the bevel tip will pierce the vial first. As downward pressure is applied, there is a slight bend in the needle, and the bevel heel will enter through the opening made by the bevel tip.
Semi-rigid Containers—When using a polyolefin, semirigid container for admixture preparation, remove the protective screw cap and add drugs through the designated injection portal. Disinfecting the portal and replacing the protective cap are not necessary, but most pharmacies swab the port as a matter of procedure.
Premixed Solutions
Bags/Bottles Containing Powder for Reconstitution. Some drugs are available in powdered form in final containers of plastic or glass. This system requires that 20 to 100 mL of sterile diluting fluid, such as 0.9% sodium chloride or sterile water for injection, are added to the bottle to reconstitute the drug. Once reconstituted and labeled by the pharmacy, these products are administered via piggyback systems.
Basic Continuous Intravenous Therapy. In the basic setup, the IV fluid is a large-volume parenteral (LVP) that is hung on an IV pole or other device approximately 36 inches higher than the patient’s bed or head. This allows the flow of IV solution to be maintained by gravity (figure 16–6).2 Attached to the LVP is a set of sterile tubing that is usually referred to as a primary IV set. The primary IV set extends down from the LVP to a catheter that has been placed in the patient’s vein. IV solution setups may differ between patients due to the infusion device or the special needs of the patient.
The LVP is usually a simple solution of dilute dextrose, sodium chloride, or both. It may contain additives, such as potassium, if the patient’s clinical condition warrants it. The solution is infused continuously to keep blood from clotting in the catheter and plugging it up. The fluid is also used to deliver drugs and to help prevent or reverse dehydration.
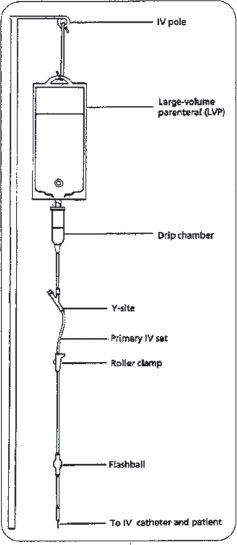
Figure 16–6. A LVP hanging on an IV pole, showing the primary IV set, including drip chamber, Y-site and flashball injection sites, and roller clamp, which can be used to control the flow of fluid.
Administration Systems for Parenteral Products
Patients receiving IV therapy usually have a basic IV setup that includes a LVP solution, or they have a catheter specifically designed for periodic injections (heparin lock, butterfly, etc.). Based on this, IV drug administration systems are typically classified as either continuous infusions or intermittent injections.
Continuous Infusions
Some drugs are administered as a continuous infusion because they are more effective and less toxic than when given intermittently. Continuous infusions include basic fluid and electrolyte therapy, blood products, and drugs that require tight administration control to minimize adverse effects.
Intermittent Injections
Intermittent injection systems are used to administer medications that work better when infused at defined time intervals rather than when infused continuously. The reason may be that periodic administration of the drug increases efficacy or reduces toxicity. Examples of drugs commonly given intermittently are antibiotics and drugs used to treat or prevent gastrointestinal ulcers (e.g., proton pump inhibitors, such as pantoprazole).
Several types of systems are available for intermittent injections. Each system has advantages and disadvantages related to cost, flexibility, waste, and so on. This section addresses how to prepare products for use with each system. Institutional policies dictate specific labeling, expiration dating, and storage conditions, and so they are not addressed here.
Commercially Available Pre-Mixed Admixtures
LVPs with additives manufactured in standard concentrations are stable in solution for longer periods of time than those compounded in the pharmacy and are available in a variety of sizes (250 mL, 500 mL, 1000 mL) and containers (glass or plastic) depending on the product and its use. Examples include lidocaine, potassium, nitroglycerin, dopamine, and aminophylline. Ready-to-use products are advantageous because they reduce handling by the pharmacy and therefore, the potential for contamination. In some cases, these agents are used for emergency situations and may be stocked in the patient care area for immediate access. Standard concentrations of IV medications can decrease potential medication errors in compounding and administration.
Pharmacy Prepared Admixtures
Some solutions are made in the pharmacy to meet the specific needs of patients. Solutions are prepared in different volumes (100 mL, 250 mL, 500 mL, or 1000 mL) and different containers (glass, plastic, bag, bottle or syringe), depending on the drug and its intended use. The preparation of admixtures in the pharmacy should follow the techniques described in the section of this chapter titled “Aseptic Preparation of Parenteral Products.”
Syringe Systems
The most common drug delivery systems that use syringes are syringe pumps, volume control chambers, gravity feed, and intravenous push systems. Syringe systems require that the pharmacy fill syringes with drugs and label them. Drug stability in syringes may differ from the stability of the same drug in other dosage forms because of concentration differences.
Syringe Pumps
Syringes can be used to administer drugs by means of a specially designed syringe pump and tubing set. The pump is adjusted to administer the desired volume from the syringe over a given period of time. Pumps are either operated by a battery or a compressed spring. Pumps are available to administer a single dose per setup or a supply at pre-programmed intervals. Many of these setups require a special small-bore tubing set that determines the rate at which the drug is administered. One important pharmacy implication is that doses must be sent from the pharmacy in standard syringe sizes and concentrations. This procedure allows doses to be administered to patients more safely because many syringe pumps are pre-programmed to deliver volumes that are based on standard concentrations.
Electronic Infusion Devices and “Smart Pumps”
Electronic infusion devices, typically categorized as either pumps or controllers, are used to increase the precision and accuracy of administration primarily of IV bags and syringes. Electronic infusion devices are usually used in fluid restricted patients or when the IV solution contains a drug that must be administered at a precise rate that cannot be ensured by using the gravity method.
The term “smart pumps” is used to refer to pumps designed to alert the user to an infusion setting that does not match a facility’s drug administration guidelines. The medication’s infusion parameters, such as dose, dosing unit (mcg/kg/min, units/hr, etc.), rate, or concentration can be safely chosen with notification for doses that fall outside the recommended range. Smart pumps also allow for updates to be sent to the pumps and pump log data to be sent to the information system via twoway communication over the hospital network. Most significantly, all smart pumps are designed to prevent unintentional overdoses of medication or fluid, referred to as free-flow protection.
Volume Control Chambers (Buretrol or Volutrol)
Syringes can be used to administer drugs through a volume control, or volumetric chamber (figure 16–7). The drug is injected through a port on top of the chamber and solution is added from the primary LVP. With this system, minimal amounts of fluid can be given per dose, a method that may be beneficial in fluid-restricted or pediatric patients. This setup allows for controlled administration of fluids, since the nurse can clamp off the solution after the volume in the chamber has infused. Since multiple drugs might be in the chamber at the same time, potential for incompatibilities and unpredictable rates of administration exist. For this reason, it is important that each medication be followed by an IV flush, usually with normal saline. A disadvantage to this system is the increased potential for infection because multiple manipulations may occur.
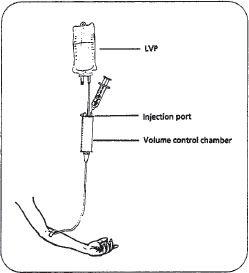
Figure 16–7. A volume control setup.
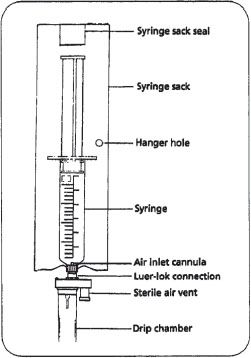
Figure 16–8. A gravity-feed syringe.
Gravity Feed
Syringes can be used to administer drugs directly by gravity if a specially designed tubing set is used (figure 16–8). The set has an air vent through which air enters the syringe as fluid is pulled out by gravity. The syringe is prepared in the pharmacy, labeled, and sent to the nurse for administration. The system is relatively inexpensive and requires no other special equipment.
Intravenous Push
Drugs given by IV push are injected directly into the IV tubing and pushed into the patient quickly (figure 16–9). With this method, the drug is injected into an injection port, a Y-site on the IV tubing, or an injection flashball. The primary IV set is usually clamped off just above the injection port so that the drug is delivered to the patient directly, resulting in the rapid onset of the drug’s effects.
This system is used in emergencies as well as more routine situations. Disadvantages of the IV push method are that it is difficult to control the rate of drug delivery with a syringe, and many drugs cause the patient to experience adverse effects when given too quickly.
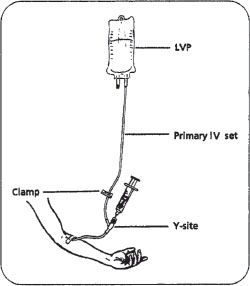
Figure 16–9. IV push setup using a Y-site.
Patient Controlled Analgesia
A method of drug administration used for injectable pain medications is patient controlled analgesia or PCA, which is very effective in managing pain. Two advantages of PCAs are that they eliminate the need for painful intramuscular injections and they give patients more control over managing their pain. The goal of PCA therapy is to relieve pain as soon as the patient recognizes a need for it. It may also reduce nursing time associated with the administration of pain medications. Patients are sometimes given a basal (or constant rate) of drug and have the ability to supplement this with a bolus or immediate dose of medication as needed.
PCA is usually administered by using either a stationary or a portable pump that infuses analgesics directly into an IV line. The pump releases a programmed amount of the pain medication into the IV tubing when the patient pushes a button. The pump is programmed to release an amount of pain medication that is specific for the patient’s weight and condition. The pump is also programmed to limit how often the patient may push the button and receive pain medication. For example, the pump may be programmed to allow a patient to receive a maximum of 1 mg of morphine sulfate every fifteen minutes. When the patient pushes the button, the pump injects 1 mg. If the patient pushes the button again in ten minutes, the pump does not release drug.
If the patient pushes the button at least five minutes later (fifteen minutes since the last injection), the pump again administers 1 mg. This is referred to as a fifteenminute lockout period.
PCA preparations may be commercially available or be prepared in the pharmacy. Preparation of these products involves the same techniques as other parenteral products. They differ from most other products in two regards. First, if the patient does not have other means of pain control, there may be an urgency to initiate therapy. Much of this urgency can be avoided with preplanning among the physician, the nurse, and the pharmacy. Second, these doses usually contain enough medication to last at least eight hours and often up to twenty-four hours or more. The result is usually a very large amount of narcotic in one container, necessitating awareness of security issues in order to prevent diversion or theft. PCA solutions may sometimes be administered subcutaneously. As with IV infusions, PCAs should never be administered without an infusion pump to protect against inadvertent overdose.
IV Locking Boxes—For infusions containing controlled substances, it is common for the final product to be hung in an IV locking box. This is a plastic box that encases the IV to prevent potential drug diversion (figure 16–10.)

Unique Infusion Devices and Containers
The delivery systems described thus far meet the needs of typical hospitalized patients. A number of new types of infusion devices and containers have been developed in the past twenty years to meet patient needs not met by traditional systems. Many of these products are designed to deliver drugs through a compact system that allows the patient to receive home infusion therapy. The system may be drugor therapy-specific, such as an implanted pump with a drug reservoir for continuous low-dose chemotherapy administration. This type of system is surgically placed under the skin and the catheter is inserted in a vein. It has a built-in power source and space for the drug solution, so that the patient does not need to carry a pump, start a new IV periodically, or need any other supplies related to the IV therapy. The downside is that it requires surgery and can only be used for drug therapies administered in very small amounts over long periods of time.
Another type of system uses an elastomeric infusion device (EID) that acts as its own pump, not requiring gravity or an electronic infusion device. These systems are similar in concept to a water balloon inside a plastic bottle. The balloon is filled with drug solution, and the pressure of the container forces it through the tubing, eliminating the need for a separate pump. These systems, whether used for hospitalized patients or home care patients, present unique challenges in filling technique, drug stability, and administration methods. Personnel preparing drugs for use in these types of devices should become familiar with the devices themselves in order to prevent errors and complications. Some of the EIDs commercially available are the Intermate® and the Homepump Eclipse® Infusions systems. Each device is labeled with a flow rate in mL/hour. The duration of the infusion is dependent on the volume of the drug solution. The concentration of drug solution may determine the volume needed.
Administration Sets
Primary IV Set. The primary IV set attached to the LVP can be one of several varieties, but the IV sets that flow by the force of gravity have several common features. The tubing has a drip chamber that is used to estimate the administration rate by counting drops as they fall through the chamber. Drip chambers are typically classified as macrodrip or minidrip based on the size of the drop that is formed in the drip chamber. Each set of tubing is labeled according to the number of drops it produces from one milliliter of solution. This number is used to determine how many drops should fall in a minute for the desired volume of milliliters per hour. Macro-drip sets deliver ten to twenty drops per milliliter and minidrip sets deliver sixty drops per milliliter, which offers precision necessary for pediatric patients.
The rate of flow through the tubing is set by use of a roller clamp rate-controlling device or an electronic infusion device. The roller clamp crimps the IV tubing as it is adjusted to control the flow of fluid. Electronic infusion devices, typically categorized as either pumps or controllers, are used to increase the precision and accuracy of administration.
The tubing may have injection ports, either Y-sites or flashballs. Drugs or other solutions can be injected through the injection ports so that they may be administered with the main IV solution. The systems used to give drugs through this means vary in setup.
Secondary IV Sets. Drugs that are routinely given through the same basic IV setup are usually attached to a “secondary IV set” that is connected to the primary set.
Venous Access Devices
Peripheral Venous Catheters are inserted into a peripheral vein (a vein of the arm, leg, hand, foot, or scalp) or a central vein (existing in the chest near the heart). Where the catheter is inserted depends, in part, on the contents of the IV solution. Peripheral insertion is more common than central insertion. With peripheral catheters, there are limitations on what can be infused and at what rate. The central catheter is more complicated and riskier to insert and maintain, but has fewer restrictions with respect to concentration of drug, rate of administration, and time the venous access can remain in place.
There are several types of peripheral catheters. The most common is plastic, because it is flexible and can bend as the vein flexes or moves and is therefore the most comfortable for the patient. Another type is a steel needle with a short end of tubing. This type is commonly referred to as a scalp vein or butterfly because of its appearance. This type of catheter may be left in the patient’s vein even without a running IV if it is periodically flushed (rinsed) with a solution to prevent it from being blocked by blood clots. It is usually used in patients that require IV therapy but are otherwise able to eat and drink, do not require supplemental fluids, and may even be ambulatory.
Central catheters can be temporary, meaning they are only used for days or weeks (such as during a hospital stay), or permanent, which can be used for months or years (such as with home care patients or cancer patients who require frequent infusions). Temporary central catheters are inserted by the physician via a minor surgical procedure in the patient’s room. This involves a small incision and insertion of the catheter into a vein near the heart. Permanent placement of central catheters also involves minor surgery but must be done in an operating room. The central catheter gives direct access into a vein that has a high flow of blood. Therefore, solutions that might be irritating or damaging to peripheral blood vessels, which have a lower blood flow, are given centrally.
Two commonly used permanent catheters are the Hickman®and the Broviac®. Each of these are tunnel catheters, which remain outside the body to provide readily available access for patients receiving multiple injections. Port-a-cath® catheters are another form of central injection port that is located beneath the skin. Another type of catheter that offers some of the benefits of both central and peripheral catheters is called a peripherally inserted central catheter (PICC). The PICC line, as its name implies, is inserted peripherally, but it is a long flexible catheter that is threaded through the venous system and its tip ends near the heart, where there is a high volume of blood flow. Caution needs to be exercised when manipulating all catheters because they can become a source of infection and can become seeded or colonized with bacteria.
Heparin Lock. Heparin locks are used to maintain catheter access to a vein without having to run a continuous drip to keep the vein patent or unobstructed. Heparin locks have an IV catheter or needle on one end and a resealable rubber diaphragm at the other end. The main purpose is to provide a port through which medications can be administered intermittently. The concentration of heparin used in heparin locks is usually 10 units/mL or 100 units/mL (figure 16–11).

Figure 16–12. Syringe with cannula.
Needleless Systems. Needleless systems are becoming a cost-effective alternative to traditional needle systems. They reduce the risks of needle sticks and subsequently the potential risk of disease transmission to healthcare workers who regularly draw blood or administer medications to patients. Needleless systems are required by law in some states and some health care systems. Needleless systems contain specially designed components, which may include a cannula or positive pressure cap that may be directly connected to a syringe tip and a needleless injection site. Cannulas functions like a needle but contain a blunt tip. The injection site must have the ability to accept the cannula (figure 16–12).
Final Filters. Final filters are inline filters located in the tubing used for drug administration. Final filters can be used to remove particles that may be present in the IV solution but are not visible to the naked eye. These particles, while small, can be harmful to patients if they become trapped in small capillaries or accumulate in the body. Final filters should be used with drugs that have a risk of particulate matter or crystals in the final solution, such as with phenytoin or mannitol.
Aseptic Preparation of Parenteral Products
As the use of parenteral therapy continues to expand, the need for well-controlled admixture preparation has also grown. Recognizing this need, many pharmacy departments have devoted increased resources to programs that ensure the aseptic preparation of sterile products. The main elements of these programs are4,5
 Development and maintenance of good aseptic technique in the personnel who prepare and administer sterile products
Development and maintenance of good aseptic technique in the personnel who prepare and administer sterile products
 Development and maintenance of a sterile compounding area, complete with sterilized equipment and supplies
Development and maintenance of a sterile compounding area, complete with sterilized equipment and supplies
 Development and maintenance of the skills needed to properly use a laminar airflow workbench (LAFW) or laminar airflow hood
Development and maintenance of the skills needed to properly use a laminar airflow workbench (LAFW) or laminar airflow hood
Aseptic Technique
Aseptic technique is a means of manipulating sterile products without compromising their sterility. Proper use of a LAFW and strict aseptic technique are the most important factors in preventing the contamination of sterile products. Thorough training in the proper use of the LAFW and strict aseptic technique, followed by the development of conscientious work habits, is of utmost importance to any sterile products program.
Sterile Compounding Area (i.e., Clean Room)
Compounded sterile products must be free of living microorganisms, pyrogens, and visible particles. Room air typically contains thousands of suspended particles per cubic foot, most of which are too small to be seen with the naked eye. These include contaminants such as dust, pollen, smoke, and bacteria. Reducing the number of particles in the air improves the environment in which sterile products are prepared. This can be done by following several practices to maintain the sterile compounding area.
Compounding area counters, easily cleanable work surfaces, and floors should be cleaned daily while walls, ceilings, and storage shelving should be cleaned monthly at a minimum.6 Segregated compounding areas must be separate from normal pharmacy operations, nonessential equipment, and other materials that produce particles. For example, the introduction of cardboard into the clean room environment should be avoided. Traffic flow into the sterile compounding area should be minimized. Trash should be removed frequently and regularly. Care should be taken to take the trash cans outside of the IV room before pulling the trash or otherwise removing it from the container. This will minimize the creation of particulate matter or the risk of spills in the IV room. Other, more sophisticated aspects of clean room design include special filtration or treatment systems for incoming air (figure 16–13), ultraviolet irradiation, air-lock entry portals, sticky mats to remove particulate matter from shoes, and positive room air pressure to reduce contaminant entry from adjacent rooms or hallways. Clean rooms are often adjoined by an anteroom that is used for non-aseptic activities related to the clean room operations, such as order processing, gowning, and handling of stock.

Figure 16–13. Ceiling HEPA filter.
Sterile products should be prepared in ISO Class 5 environments, which contain no more than 100 particles per cubic foot that are 0.5 micron or larger in size. LAFWs are frequently used to achieve an ISO Class 5 environment.
Laminar Airflow Workbenches
The underlying principle of LAFWs is that twice-filtered laminar layers of aseptic air continuously sweep the work area inside the hood to prevent the entry of contaminated room air. There are two common types of laminar flow workbenches, horizontal flow and vertical flow.
Horizontal LAFW. LAFWs that sweep filtered air from the back of the hood to the front are called horizontal LAFWs (figure 16–13). Horizontal flow workbenches use an electrical blower to draw contaminated room air through a prefilter. The prefilter, which is similar to a furnace filter, only removes gross contaminants and should be cleaned or replaced on a regular basis. The prefiltered air is then pressurized in a plenum to ensure that a consistent distribution of airflow is presented to the final filtering apparatus. The final filter constitutes the entire back portion of the hood’s work area. This high efficiency particulate air, or HEPA filter, removes 99.97% of particles that are 0.3 micron or larger, thereby eliminating airborne microorganisms, which are usually 0.5 microns or larger.
Vertical LAFW. Laminar flow workbenches with a vertical flow of filtered air are also available. In vertical LAFWs, HEPA filtered air emerges from the top and passes downward through the work area (figure 16–14). Because exposure to antineoplastic (anticancer) drugs may be harmful, they should only be prepared in vertical LAFWs so that the risk of exposure to airborne drug particulates is minimized. The types of vertical LAFW used for the preparation of antineoplastics confine airflow within the hood and are referred to as biological safety cabinets (BSCs). BSCs and the preparation of antineoplastics and cytotoxic medications are covered later in this chapter.
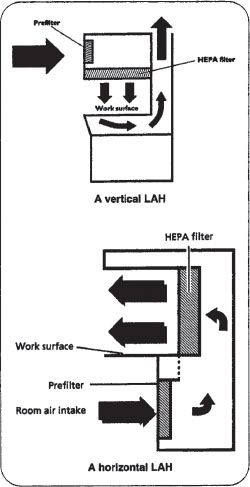
Figure 16–14. Horizontal and vertical laminar flow hoods with the basic components labeled.
The critical principle of using LAFWs is that nothing should interrupt the flow of air between the HEPA filter and the sterile object. The space between the HEPA filter and the sterile object is known as the critical area. The introduction of a foreign object between a sterile object and the HEPA filter increases wind turbulence in the critical area and contaminants from the foreign object may be carried onto the sterile work surface and thereby contaminate the injection port, needle, or syringe. This is referred to as downstream contamination.
 To maintain sterility, nothing should pass behind a sterile object in a horizontal flow workbench or above a sterile object in a vertical flow workbench, including the technician’s hands.
To maintain sterility, nothing should pass behind a sterile object in a horizontal flow workbench or above a sterile object in a vertical flow workbench, including the technician’s hands.
All materials placed within the laminar flow workbench disturb the patterned flow of air blowing from the HEPA filter. This zone of turbulence created behind an object could potentially extend outside the hood, pulling or allowing contaminated room air into the aseptic working area (figure 16–15). When laminar airflow is moving on all sides of an object, the zone of turbulence extends approximately three times the diameter of that object. When laminar airflow is not accessible to an object on all sides (for example, when placed adjacent to a vertical wall), a zone of turbulence is created that may extend six times the diameter of the object. For these reasons, the hands should be positioned so that airflow in the critical area between the HEPA filter and sterile objects is not blocked.
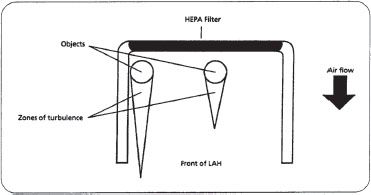
Figure 16–15. Examples of zones of turbulence created behind objects in a horizontal LAFW. Notice that the zone of turbulence of the object on the left is greater due to the object’s proximity to the side of the hood, and has extended outside of the LAFW. (Note: figure is not drawn to scale.)
 It is advisable to work with objects at least six inches from the sides and front edge of the hood without blocking air vents, so that unobstructed airflow is maintained between the HEPA filter and sterile objects.
It is advisable to work with objects at least six inches from the sides and front edge of the hood without blocking air vents, so that unobstructed airflow is maintained between the HEPA filter and sterile objects.
The following are general principles for operating LAFWs properly:
 A LAFW should be positioned away from excess traffic, doors, air vents, or anything that could produce air currents capable of introducing contaminants into the hood.
A LAFW should be positioned away from excess traffic, doors, air vents, or anything that could produce air currents capable of introducing contaminants into the hood.
 If it is turned off, nonfiltered, nonsterile air will occupy the LAFW work area. Therefore, when it is turned back on, it should be allowed to run for fifteen to thirty minutes before it is used. Manufacturer recommendations should be consulted for each given hood. This allows the LAFW to blow the non-sterile air out of the LAFW work area. Then the LAFW can be cleaned for use.
If it is turned off, nonfiltered, nonsterile air will occupy the LAFW work area. Therefore, when it is turned back on, it should be allowed to run for fifteen to thirty minutes before it is used. Manufacturer recommendations should be consulted for each given hood. This allows the LAFW to blow the non-sterile air out of the LAFW work area. Then the LAFW can be cleaned for use.
 Before use, all interior working surfaces of the laminar flow workbench should be cleaned with 70% isopropyl alcohol or other appropriate disinfecting agent and a clean, lint-free cloth.
Before use, all interior working surfaces of the laminar flow workbench should be cleaned with 70% isopropyl alcohol or other appropriate disinfecting agent and a clean, lint-free cloth.
 Cleaning should be performed from the HEPA filter beginning in the rear of the hood in a side-to-side motion parallel to that surface’s contact with the HEPA, moving gradually away from the point of contact in overlapping strokes. Cleaning should never be done with a motion that carries contaminants from the outer edge back toward the HEPA.
Cleaning should be performed from the HEPA filter beginning in the rear of the hood in a side-to-side motion parallel to that surface’s contact with the HEPA, moving gradually away from the point of contact in overlapping strokes. Cleaning should never be done with a motion that carries contaminants from the outer edge back toward the HEPA.



