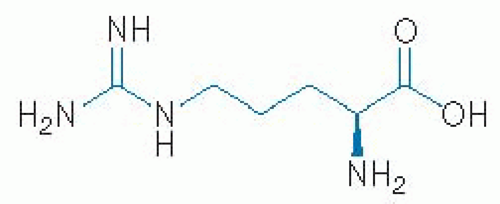Arginine, Citrulline, and Nitric Oxide1
Yvette C. Luiking
Leticia Castillo
Nicolaas E.P. Deutz
1Abbreviations: ADMA, asymmetric dimethylarginine; ASL, argininosuccinate lyase; ASS, argininosuccinate synthase; BH4, tetrahydrobiopterin; CAT, cationic amino acid transporter; DDAH, dimethylaminohydrolase; EDRF, endothelium-derived relaxing factor; HIV, human immunodeficiency virus; IL, interleukin; LPS, lipopolysaccharide; NO, nitric oxide; NOx, nitrate and nitrite; NOS, nitric oxide synthase; OTC, ornithine transcarbamoylase.
HISTORICAL INTRODUCTION
Arginine is a semiconditional or conditional essential amino acid, which implies that healthy adults have no specific nutritional need for arginine. In neonates, in infants, and in certain conditions, however, endogenous arginine synthesis is not sufficient to meet the requirements; this deficiency can be related to insufficient synthesis of arginine precursors such as citrulline. Beyond protein synthesis, arginine is a metabolite in the urea cycle and is a well-known substrate for ureagenesis in the liver. In the 1980s, an endothelium-derived relaxing factor (EDRF) was found in endothelial cells (1). EDRF was subsequently identified as nitric oxide (NO) with L-arginine as its precursor (2), thus enhancing the functional relevance of arginine. Investigators also became increasingly aware that NO is a ubiquitous molecule, present in cardiovascular and nervous system cells as well as in inflammatory cells, with many physiologic functions and pathophysiologic implications (3, 4, 5).
Citrulline is a nonprotein amino acid, a characterization implying that it is not used in protein synthesis. Its name is derived from the Latin Citrullus vulgaris, which means watermelon, from which it was first isolated in 1930s. The relevance of citrulline was long neglected because citrulline was largely seen as an intermediary of the urea cycle. This perception changed, however, as a result of work on the interorgan exchange of citrulline and the identification of citrulline as a precursor for de novo arginine synthesis (6). More recently, the identification of plasma citrulline as a biomarker of intestinal functional mass (7) and evidence of the direct action of citrulline as a promoter of muscle protein synthesis (8) have added to our understanding of citrulline’s biologic relevance. Citrulline is now suggested as a conditional essential amino acid, at least in persons with disorders characterized by compromised intestinal function (9, 10, 11).
METABOLISM AND FUNCTION IN HEALTH
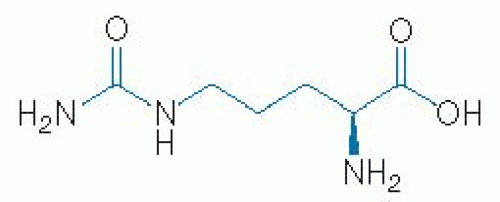
L-Arginine is a basic amino acid. Its structure is illustrated in Figure 35.1. L-Arginine has a molar mass of 174.2 g/mol and is characterized by a guanidino group. Citrulline is an α-amino acid. Its structure is illustrated in Figure 35.2. Citrulline has a molar mass of 175.19 g/mol and is characterized by a ureido group (10).
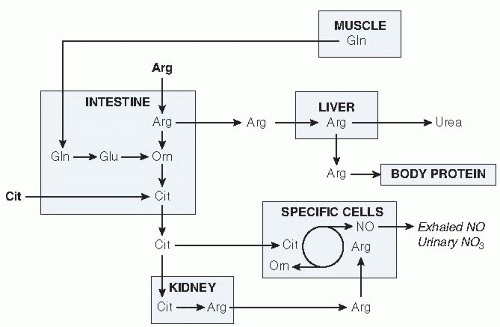
The metabolism of arginine and citrulline can roughly be divided into a synthesis pathway and a utilization or catabolic pathway, with interorgan exchange of metabolites (Figs. 35.3 and 35.4). Pharmacokinetic studies indicate that citrulline is relatively better absorbed and has higher systemic bioavailability than arginine (12).
Arginine
Arginine Synthesis Pathway
Arginine is mainly available from protein breakdown in the body and from food intake. The jejunum is the major site for intestinal absorption of dietary arginine. Only approximately 20% of protein synthesis is derived directly from dietary
amino acid intake. This finding implies that approximately 80% of protein synthesis involves recycling of amino acids from protein breakdown. Moreover, arginine is synthesized endogenously or de novo in the proximal renal tubule by conversion of citrulline to arginine through a partial urea cycle by the enzymes argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL) (13, 14, 15, 16). This conversion is part of the intestinal-renal axis, as demonstrated in animal and human studies (6, 17, 18, 19). Under normal conditions, this pathway contributes approximately 10% to 15% to whole body arginine production (20, 21), in which citrulline availability is the limiting factor for renal arginine synthesis (15). In contrast to adults, the conversion to arginine in neonates is limited to intestinal arginine synthesis from dietary proline and the conversion of citrulline to arginine by the enzymes ASS and ASL (22). This first-pass de novo synthesis provides 50% of the arginine needed by neonates (23). In the postabsorptive state, whole body arginine flux in healthy adults is approximately 70 to 90 µmol/kg/hour (24).
amino acid intake. This finding implies that approximately 80% of protein synthesis involves recycling of amino acids from protein breakdown. Moreover, arginine is synthesized endogenously or de novo in the proximal renal tubule by conversion of citrulline to arginine through a partial urea cycle by the enzymes argininosuccinate synthase (ASS) and argininosuccinate lyase (ASL) (13, 14, 15, 16). This conversion is part of the intestinal-renal axis, as demonstrated in animal and human studies (6, 17, 18, 19). Under normal conditions, this pathway contributes approximately 10% to 15% to whole body arginine production (20, 21), in which citrulline availability is the limiting factor for renal arginine synthesis (15). In contrast to adults, the conversion to arginine in neonates is limited to intestinal arginine synthesis from dietary proline and the conversion of citrulline to arginine by the enzymes ASS and ASL (22). This first-pass de novo synthesis provides 50% of the arginine needed by neonates (23). In the postabsorptive state, whole body arginine flux in healthy adults is approximately 70 to 90 µmol/kg/hour (24).
Arginine Catabolic Pathway
Apart from being an essential component of body proteins, arginine plays a key role in several other metabolic pathways that involve various enzyme systems (3, 4, 12, 25, 26, 27), as follows:
The arginase pathway is quantitatively the most important. Fifteen percent of arginine flux will enter this pathway (20). This finding implies the degradation of arginine to ornithine and urea by the enzyme arginase, of which two isoforms are known (arginase type I and type II). Type I cytosolic arginase is expressed in the liver, as part of the urea cycle. A full urea cycle is present only in the liver and implies detoxification of ammonia and urea synthesis through five reaction steps to excrete excess nitrogen from the body. Type II mitochondrial arginase is expressed at low levels in extrahepatic tissues and cells (e.g., brain, kidney, small intestine, red blood cells, and immune cells) and is involved in the synthesis of ornithine, proline, and glutamate (28, 29). Through ornithine and derived polyamines (putrescine, spermine, and spermidine), arginine is important for cell growth and differentiation (30). By means of proline, which is hydroxylated to form hydroxyproline, arginine is involved in collagen formation, tissue repair, and wound healing (31). Approximately 40% of arginine that is absorbed from the intestinal lumen is degraded in the first pass (32) because of relatively high arginase activity in the intestinal mucosa.
Arginine is converted to NO by three isoforms of the enzyme NO synthase (NOS), with concomitant formation of citrulline (33, 34). Approximately 1.5% of arginine flux enters this pathway (20). NOS-1 (neuronal NOS) and NOS-3 (endothelial NOS) enzymes produce NO that acts as a neurotransmitter and as a vasodilator, respectively (34). NO synthesized by NOS-2 (inducible NOS) at high levels has immune regulatory functions, such as control or killing of infectious pathogens, modulation of cytokine production, and T-helper cell
development. Moreover, this NO derived from NOS-2 acts cytoprotective as a free radical scavenger (35) when it is induced by elevated circulating cytokine concentrations (mainly tumor necrosis factor-α, and interleukin [IL]-1, IL-6, and IL-8) or microbial products (e.g., lipopolysaccharide [LPS]) during inflammatory processes (33, 34, 36, 37, 38). This property has led to the suggestion that arginine could have a great potential as an immune modulator (39, 40), and it may prove useful in enhancing the immune response in various models of immunologic challenges (41).
A large amount of arginine (˜10% of arginine flux, equal to ˜2.3 g arginine/day in humans) is used for the biosynthesis of creatine through the interorgan cooperation of kidneys, pancreas, liver, and skeletal muscle (27). Creatine is an important constituent of skeletal muscle and neurons and acts as an energy source for these tissues. Creatine is excreted in urine as creatinine (27).
Finally, agmatine is a decarboxylation product of arginine and acts as a cell signaling molecule (3).
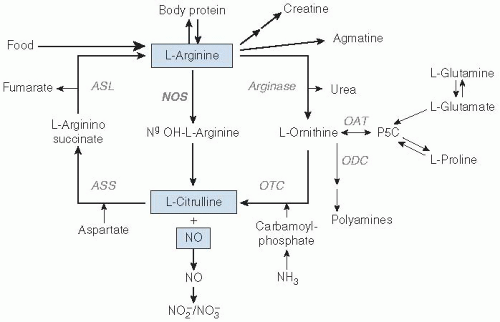 Fig. 35.3. Metabolic pathway of arginine, citrulline, and nitric oxide (NO). In this schematic overview of arginine, citrulline, and NO metabolism, arginine is derived from food, body protein, and de novo synthesis from citrulline. Arginine is the substrate for synthesis of body proteins, NO and citrulline, urea and ornithine, creatine, and agmatine. Citrulline is derived from food (minor amount) and from endogenous synthesis from glutamine and arginine. ASL, argininosuccinate lyase; ASS, argininosuccinate synthase; NO, nitric oxide, NOS, nitric oxide synthase; OAT, ornithine aminotransferase; ODC, ornithine decarboxylase; OTC, ornithine transcarbamoylase; P5C, pyrolline-5-carboxylate. (Data with permission from references 24, 27, and 86.) |
Other Direct Actions of Arginine
In addition to its role as an intermediary in the synthesis of functional products, arginine also acts as a secretagogue because it stimulates release of several hormones such as insulin, glucagon, somatostatin, prolactin, growth hormone, and its peripheral mediator, insulinlike growth factor-I (30, 42). Arginine has the strongest insulinogenic effect of all the amino acids (27).
Citrulline
Citrulline Synthesis Pathway
Citrulline is synthesized by enterocytes in the small intestine that convert glutamine and proline through the glutamate-to-ornithine pathway (43). The final step in this synthesis pathway is the conversion of ornithine to citrulline, catalyzed by the enzyme ornithine transcarbamoylase (OTC) or ornithine carbamoyltransferase. Aside from the liver, where OTC is an enzyme in the urea cycle, OTC is present only in enterocytes (27). Glutamine is considered the main precursor for citrulline synthesis, as demonstrated by the close relationship between glutamine uptake and citrulline release by the intestine (44), which provides 60% to 80% of citrulline (19, 45, 46, 47, 48). Moreover, arginine has been suggested as a source of citrulline through arginase and OTC metabolic pathways (49), and interorgan exchange of ornithine may also contribute to citrulline synthesis in the intestine (50). An additional amount of citrulline comes from nonintestinal sources. The intracellular arginine-citrulline cycle related to NO production in endothelial cells seems a likely candidate, as suggested in mice, humans, and endothelial cell studies (18, 46, 51). In the postabsorptive state, whole body citrulline flux in healthy adults is approximately 10 to 15 µmol/kg/hour (52, 53).
Citrulline Catabolic Pathway
Unlike most amino acids, citrulline is not incorporated into protein, but it can be converted to only arginine.
A large part of circulating citrulline, which is partly derived from the intestinal release of citrulline, is taken up by the kidneys (6, 17), where it is converted to arginine that is released into the circulation. This pathway has been confirmed in humans (18, 19). Although investigators thought that citrulline thus escaped the splanchnic sequestration and bypassed the urea cycle with subsequent nitrogen loss (30), other investigators indicated that the liver does extract substantial amounts of citrulline from the portal vein (18). Moreover, citrulline conversion to arginine is also efficient in other cells such as macrophages, especially under low-arginine conditions (54). This gives citrulline a considerable role in metabolism and regulation of NO (10).
A large part of circulating citrulline, which is partly derived from the intestinal release of citrulline, is taken up by the kidneys (6, 17), where it is converted to arginine that is released into the circulation. This pathway has been confirmed in humans (18, 19). Although investigators thought that citrulline thus escaped the splanchnic sequestration and bypassed the urea cycle with subsequent nitrogen loss (30), other investigators indicated that the liver does extract substantial amounts of citrulline from the portal vein (18). Moreover, citrulline conversion to arginine is also efficient in other cells such as macrophages, especially under low-arginine conditions (54). This gives citrulline a considerable role in metabolism and regulation of NO (10).
Other Direct Actions of Citrulline
DIETARY SOURCES AND NUTRITIONAL NEEDS
The major nutritional sources of arginine are dietary proteins. The amount of arginine is relatively high in seafood, nuts, seeds, algae, meats, rice protein concentrate, and soy protein isolate. The milk of most mammals (including cows, humans, and pigs) is relatively low in arginine (4). Daily dietary arginine intake in healthy individuals is approximately 4 to 6 g (42, 56), but 25% of the US adult population consumes less than 2.6 g/day (57). This dietary arginine intake seems minor, however, compared with the whole body arginine flux of approximately 15 to 20 g/day (20, 53).
Apart from watermelon, in which citrulline can be found in small quantities (1 g of citrulline in 780 g of watermelon), citrulline intake through food is nearly absent (10). No recommended dietary allowances are available for either arginine or citrulline.
FACTORS THAT INFLUENCE UTILIZATION AND METABOLISM
Several factors can modulate arginine, citrulline, and NO metabolism. These factors can be either endogenous (intrinsic) or exogenous (extrinsic).
Endogenous Influencing Factors
Endogenous factors that modulate arginine, citrulline, and NO metabolism are compartmentalization of metabolism, intracellular transport systems, coupling among enzymes, competition among enzymes that convert arginine to its metabolites, and endogenous NOS inhibitors.
Compartmentalization of Metabolism
The reason for compartmentalization of metabolism is that the enzymes in arginine-citrulline metabolism are expressed to a different extent in various organs (27, 58), and interorgan exchange occurs (see Fig. 35.4) (44). The direct conversion of citrulline to arginine and subsequently to NO (citrulline-NO cycle) in macrophages (59) or endothelial cells (51) and urea metabolism in the liver or compartmentalized NO production from protein-derived arginine (50) are examples of compartmentalization.
Intracellular Transport Systems
Substrate availability for arginine-requiring catabolic enzymes also depends on arginine transport systems. Several arginine transporters exist, of which system y+ is the most important and high-affinity transport mechanism, ascribed on the molecular level to cationic amino acid transporters (CATs). Of these CATs, CAT-1, CAT-2(B), and CAT-3 have been identified, all of which differ in their tissue distribution (27). These transport systems are often colocalized with the catabolic enzymes and as such can modulate cellular arginine metabolism (27). For example, CAT-1 arginine transporter and endothelial NO synthase enzyme are colocalized in plasma membrane caveolae (60), which facilitate specific channelling of arginine to NO production without mixing with the total intracellular pool (58). Lysine, ornithine, and certain endogenous NOS inhibitors use the same transporter as arginine and may thereby compete for transporter capacity in conditions of low arginine (58, 61). For citrulline, no evidence indicates the presence of a specific transporter in any cell type, and transport by the usual generic amino acid transporters is demonstrated (10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree