Apocrine Carcinoma
EDI BROGI
Apocrine glands are normal appendages of the skin in the axilla, anogenital region, and eyelids, where they may give rise to cutaneous apocrine carcinoma.1,2,3 Benign and malignant apocrine lesions can also occur in the breast.
The origin of the apocrine cells in the breast is a subject of long-standing debate. The term “apocrine metaplasia” that is used for benign apocrine cells commonly found in the breast implies that they develop from benign mammary epithelial cells through a metaplastic process.4 Some authors have proposed that apocrine cells are degenerated5 or terminally differentiated epithelial cells.6,7 Cells with functional apocrine characteristics have been detected in fetal breast tissue.8 This observation suggests that apocrine differentiation occurs in a subset of normal mammary glandular cells and that apocrine proliferative lesions found in the adult breast may arise by expansion of this constituent rather than through metaplastic alteration of nonapocrine cells.9
Recent molecular studies suggest that mammary carcinomas with apocrine differentiation constitute a distinct subset of estrogen receptor (ER)-negative, androgen receptor (AR)-positive mammary neoplasm.10,11
Apocrine cells are characterized by abundant cytoplasm that can be densely eosinophilic, granular, or vacuolated, and by large nuclei with prominent nucleoli. These morphologic features are present to some degree in a substantial proportion of mammary carcinomas, but the term “apocrine carcinoma” should be reserved for neoplasms in which all or nearly all of the epithelium has apocrine morphology. Apocrine carcinomas have the same ER-negative and AR-positive immunophenotype as apocrine metaplasia.10,12 Further refinement of the diagnostic criteria is needed to achieve a more consistent definition of mammary apocrine lesions that integrates morphologic, immunophenotypical, and genetic data.
In this chapter, we review the literature on mammary apocrine carcinomas, specifying whether the data that are presented pertain to case series selected based on morphologic and/or immunohistochemical criteria.
CLINICAL PRESENTATION
Incidence, Age, and Gender
The frequency of apocrine carcinoma ranges from less than 1%13,14 to about 4%.9,15 This variability is probably the result of inconsistent diagnostic criteria and variations in the way cases were identified. When the diagnosis is limited to carcinomas that have apocrine morphology throughout or in most of the tumor, no more than 1% of breast carcinomas can be classified as apocrine carcinomas. In a series by Dellapasqua et al.,16 72 women with invasive carcinomas with apocrine morphology represented 1% of 6,971 patients with invasive carcinoma surgically treated at one Italian institution between 1997 and 2005. Tanaka et al.15 identified 61 patients with apocrine breast carcinomas (57 invasive and 4 ductal carcinoma in situ [DCIS]) (2.9%) among 2,055 patients with breast carcinoma treated surgically at their center over a period of 10 years.
The reported age at diagnosis of apocrine carcinoma ranges from 19 to 90 years. Most patients tend to be postmenopausal and 5 to 10 years older than patients with nonapocrine ductal carcinomas.13,16,17,18,19,20 Tanaka et al.15 reported that the mean age of 57 patients with invasive apocrine carcinoma was 58.5 ± 10.9 years, significantly higher than the mean age of 54.4 ± 11 years for 1,583 women with invasive nonapocrine ductal carcinoma treated consecutively at a Japanese institution during the same period.
Genetic Predisposition
Data on risk factors for breast carcinoma such as family history are very limited in women with apocrine carcinoma. Six patients in one series gave a negative family history for
breast carcinoma.13 Breast lesions that occur in patients with Cowden syndrome often have apocrine morphology.23,24 Banneau et al.25 reported that the molecular signature of carcinomas in patients with PTEN germline mutation (Cowden disease) had significant overlap with that of molecular apocrine carcinoma. The apocrine Cowden carcinomas included 4/15 (27%) carcinomas with apocrine morphology, 2 (13%) invasive ductal carcinomas with apocrine features, and 1 (6.6%) invasive micropapillary apocrine carcinoma. The nonapocrine Cowden carcinomas included 6/15 (40%) invasive ductal carcinomas not otherwise specified (NOS), 1 (6.6%) invasive lobular carcinoma, and 1 (6.6%) DCIS. Apocrine carcinoma is not specifically linked to Cowden syndrome.
breast carcinoma.13 Breast lesions that occur in patients with Cowden syndrome often have apocrine morphology.23,24 Banneau et al.25 reported that the molecular signature of carcinomas in patients with PTEN germline mutation (Cowden disease) had significant overlap with that of molecular apocrine carcinoma. The apocrine Cowden carcinomas included 4/15 (27%) carcinomas with apocrine morphology, 2 (13%) invasive ductal carcinomas with apocrine features, and 1 (6.6%) invasive micropapillary apocrine carcinoma. The nonapocrine Cowden carcinomas included 6/15 (40%) invasive ductal carcinomas not otherwise specified (NOS), 1 (6.6%) invasive lobular carcinoma, and 1 (6.6%) DCIS. Apocrine carcinoma is not specifically linked to Cowden syndrome.
Symptoms and Imaging
There are no striking differences in the clinical or mammographic features of patients with apocrine and nonapocrine duct carcinomas.26 Most patients with invasive apocrine carcinoma present with a mass. Pain, Paget disease, nipple discharge, and other symptoms are relatively uncommon initial manifestations of invasive or in situ apocrine carcinoma. Occasionally, apocrine DCIS is sufficiently abundant to form a mass lesion,27 but more often it is detected by mammography. When compared with other types of breast carcinoma, the distribution of sites of apocrine carcinoma does not differ from that of most breast carcinomas, with the majority of the lesions located in the upper outer quadrant. The stage at diagnosis of apocrine carcinoma is also not appreciably different from that of nonapocrine carcinomas.
Twenty-eight of 29 triple negative apocrine carcinomas in one series20 were unifocal. Patients with apocrine breast carcinoma sometimes have a contralateral nonapocrine carcinoma.28 One study16 found that patients with morphologically and immunophenotypically pure apocrine carcinomas had an increased risk of contralateral breast carcinoma (hazard ratio [HR], 4.12; 95% confidence interval [CI], 1.22 to 14) (p = 0.02). Bilateral apocrine carcinomas are very uncommon.14 Schmitt et al.29 studied simultaneous bilateral apocrine carcinomas found in a 74-year-old patient and demonstrated that they were independent primary tumors. The right breast invasive apocrine carcinoma was human epidermal growth factor 2 (HER2)-negative and p53-positive and exhibited a p53 mutation. The left breast invasive apocrine carcinoma was HER2-positive and showed no p53 reactivity or gene mutation. Moritani et al.30 evaluated the relationship of DCIS and sclerosing adenosis (SA) in a series of 24 cases. They encountered one patient with high-grade apocrine DCIS secondarily involving apocrine SA in one breast and non-apocrine DCIS and SA separately located in the contralateral breast.
GROSS PATHOLOGY
Apocrine carcinoma has no specific gross morphologic features. Invasive carcinomas are firm-to-hard tumors that usually have infiltrating borders. The bisected tumor is generally gray or white. The tan-to-brown color found in some cellular benign apocrine lesions is generally not evident in apocrine carcinomas. Exceptional tumors are grossly cystic or have a medullary appearance (Fig. 19.1). Comedo necrosis can occur in apocrine DCIS.
MICROSCOPIC PATHOLOGY
Malignant Apocrine Histology
The distinguishing feature of apocrine carcinoma is the cytologic appearance of the tumor cells. More than 80 years ago, Lee et al.31 pointed out that apocrine carcinomas “have much the same structure as other mammary carcinomata; for example, we find that the bulky adenocarcinomata, the comedocarcinomata, the papillary, intraductal and intracystic carcinomata, the carcinoma simplex, and even scirrhous carcinomata of the breast are represented in this group.” Apocrine cytomorphology has also been noted in mucinous carcinoma,9 in duct carcinomas with tubular features,13,32 in medullary carcinoma,33 and in invasive lobular carcinoma.9,34 Lin et al.35 studied a series of HER2-positive invasive breast carcinomas and identified apocrine features in 67/96 (69.8%) invasive ductal carcinomas NOS, in 2/3 (66.7%) mucinous carcinomas, in 1/2 (50%) signet ring cell carcinomas, and in 1 (100%) invasive pleomorphic lobular carcinoma.
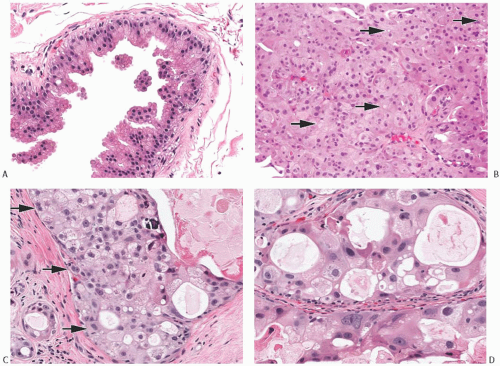
Cytologic features that characterize intraductal and invasive apocrine carcinomas are manifested in the nuclei and in the cytoplasm. The nuclei are enlarged and pleomorphic when compared with the nuclei of benign apocrine cells (Fig. 19.2). Nuclear membranes tend to be hyperchromatic and irregular. Nuclear grade can be determined within the spectrum of cytologic features found in apocrine carcinoma. Low-grade nuclei are usually slightly larger than nuclei in apocrine metaplasia and also darker due to denser chromatin. Nucleoli are usually present but inconspicuous
(Fig. 19.2). High-grade nuclei feature diverse appearances (Figs. 19.2 and 19.3). Some nuclei are strikingly enlarged and pleomorphic with one or more macronucleoli that can be round, oval, or teardrop shaped. Other high-grade apocrine nuclei have deeply basophilic and smudged chromatin in which little or no internal structure can be discerned. In other instances, the chromatin is coarse and obscures the nucleoli. Binucleation is common. Variation in nuclear size is frequently observed, with adjacent nuclei showing threefold or greater difference in diameter.
(Fig. 19.2). High-grade nuclei feature diverse appearances (Figs. 19.2 and 19.3). Some nuclei are strikingly enlarged and pleomorphic with one or more macronucleoli that can be round, oval, or teardrop shaped. Other high-grade apocrine nuclei have deeply basophilic and smudged chromatin in which little or no internal structure can be discerned. In other instances, the chromatin is coarse and obscures the nucleoli. Binucleation is common. Variation in nuclear size is frequently observed, with adjacent nuclei showing threefold or greater difference in diameter.
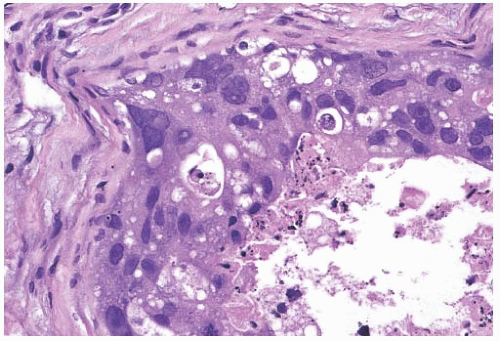
In most cases, the cytoplasm exhibits eosinophilia that is densely homogeneous or slightly granular. Cytoplasmic vacuolization or clearing is a feature associated with atypical apocrine proliferations, and it is usually most prominent in apocrine carcinomas. The cytoplasm of some tumor cells occasionally has a light blue mucoid quality, and focal mucinous secretion may be present in the lumen of the neoplastic glands, but not in the stroma. Cell borders tend to be well defined (Fig. 19.4). Some apocrine carcinomas, especially the clear cell variant, attract an intense lymphocytic or lymphoplasmacytic reaction.
Invasive Apocrine Carcinoma
Invasive apocrine carcinomas can have any of the usual growth patterns of infiltrating duct carcinoma, but they tend to be structurally poorly differentiated (Figs. 19.4 and 19.5). In two studies, 40%16 and 83%18 of invasive carcinomas with
apocrine morphology were high grade. Apocrine carcinomas typically consist of medium-sized to small tumor nests. Stromal desmoplasia and moderate to abundant lymphocytic infiltrates are common in high-grade tumors. An uncommon variant of invasive apocrine carcinoma is composed of large polygonal cells with abundant foamy or eosinophilic cytoplasm and histiocytoid morphology (Fig. 19.6).36 The presence of gross cystic disease fluid protein 15 (GCDFP-15), a marker of apocrine differentiation (see section on immunohistochemistry), has been demonstrated in these lesions by immunohistochemistry (IHC) and by in situ hybridization.36 These carcinomas can be distinguished from invasive pleomorphic lobular carcinoma by being E-cadherin positive. At present, there is insufficient follow-up information to define the prognosis of the histiocytoid type of apocrine carcinoma.
apocrine morphology were high grade. Apocrine carcinomas typically consist of medium-sized to small tumor nests. Stromal desmoplasia and moderate to abundant lymphocytic infiltrates are common in high-grade tumors. An uncommon variant of invasive apocrine carcinoma is composed of large polygonal cells with abundant foamy or eosinophilic cytoplasm and histiocytoid morphology (Fig. 19.6).36 The presence of gross cystic disease fluid protein 15 (GCDFP-15), a marker of apocrine differentiation (see section on immunohistochemistry), has been demonstrated in these lesions by immunohistochemistry (IHC) and by in situ hybridization.36 These carcinomas can be distinguished from invasive pleomorphic lobular carcinoma by being E-cadherin positive. At present, there is insufficient follow-up information to define the prognosis of the histiocytoid type of apocrine carcinoma.
Tanaka et al.15 documented lymphatic invasion in 10/57 (17.5%) apocrine carcinomas compared with 502/1,583 (31.7%) invasive nonapocrine ductal carcinomas (p = 0.02); however, other investigators have reported high incidence of lymphovascular invasion in apocrine carcinomas. Peritumoral lymphovascular invasion was present in 35%,16 38%,14 and 56%37 of cases in three series. D’Amore et al.38 encountered dermal invasion in 7/34 (21%) of their cases, including 4 (12%) with lymphatic invasion within the breast. A review of mammary carcinomas that recurred in an inflammatory pattern revealed that 33% of the primary tumors were apocrine carcinomas.39 When examined retrospectively, the majority of the primary lesions had peritumoral lymphatic tumor emboli. It is notable that apocrine carcinoma,
an uncommon tumor, was relatively more frequent than expected among patients who experienced recurrent carcinoma with an inflammatory pattern.
an uncommon tumor, was relatively more frequent than expected among patients who experienced recurrent carcinoma with an inflammatory pattern.
Apocrine Ductal Carcinoma In Situ
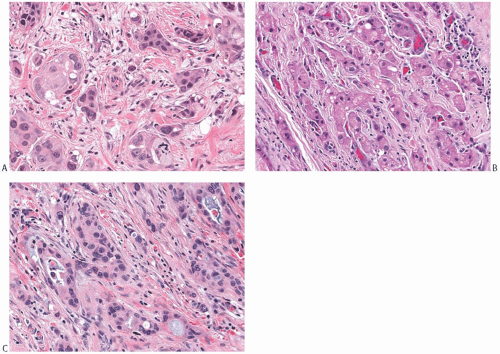
The architecture of apocrine DCIS is similar to that commonly found in nonapocrine intraductal carcinomas including comedo, micropapillary (Fig. 19.7), solid (Fig. 19.8), cribriform (Fig. 19.9), and papillary (Fig. 19.10) patterns. Apocrine DCIS with low nuclear grade can sometimes be difficult to differentiate from apocrine metaplasia. Although the cytologic atypia of low-grade apocrine DCIS can be minimal, the carcinoma shows expansive solid growth and/or complex and rigid architectural patterns characteristic of other forms of low-grade DCIS. At the other end of the spectrum, apocrine DCIS with high nuclear grade has marked nuclear pleomorphism, prominent and often irregular or multiple nucleoli, and usually shows necrosis. High-grade apocrine DCIS closely resembles nonapocrine DCIS of the same grade, but differs from the latter because it has more abundant eosinophilic, granular, or vacuolated cytoplasm. Apocrine DCIS with dense cytoplasmic eosinophilia may resemble squamous DCIS, but lacks keratin formation. Apocrine DCIS with intermediate nuclear grade usually shows obvious apocrine cytology.
Calcifications may be seen in the affected ducts. They are typically small and punctate in low-grade DCIS, but coarse, heterogeneous, and often associated with necrosis in high-grade DCIS. Periductal fibrosis and inflammation are common reactive changes around the ducts involved by apocrine DCIS with intermediate or high nuclear grade. Foamy histiocytes may be a prominent feature of the reactive changes in the stroma around apocrine DCIS40 and should not be mistaken for invasive carcinoma cells. In some cases, apocrine DCIS arises in complex papillary lesions in which there is also a benign hyperplastic component (Fig. 19.11).
Extension of apocrine DCIS into the epithelium of lobules is frequently present (Fig. 19.12).
Apocrine atypia often occurs in SA. Moritani et al.30 studied the topographic, morphologic, and immunophenotypical features of DCIS confined to SA (type A), or involving SA as well as the adjacent breast parenchyma (type B). One of 13 (7%) type A DCIS was a high-grade solid and comedotype apocrine carcinoma, with ER-negative, progesterone receptor (PR)-negative, and HER2-positive immunoprofile. The DCIS spanned 1.6 cm and was contained in a 4.5-cm focus of SA. In contrast, 5/11 (45%) type B DCIS had apocrine morphology. All five apocrine DCIS had high nuclear grade, and at least focal cribriform architecture. The second most common architectural pattern was solid in three cases.
The size of type B apocrine DCIS ranged from 2.7 to 4.2 cm, whereas the associated SA measured from 0.6 to 4.0 cm. All five type B apocrine DCIS were ER– and PR-negative; two cases were HER2-positive and two HER2-negative; the remaining DCIS had 2+ HER2 staining.
The size of type B apocrine DCIS ranged from 2.7 to 4.2 cm, whereas the associated SA measured from 0.6 to 4.0 cm. All five type B apocrine DCIS were ER– and PR-negative; two cases were HER2-positive and two HER2-negative; the remaining DCIS had 2+ HER2 staining.
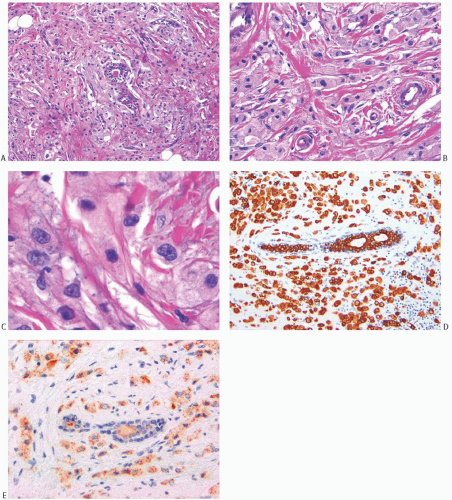 FIG. 19.6. Invasive apocrine carcinoma, histiocytoid variant. A,B: Hematoxylin and eosin (H&E)-stained samples of invasive apocrine carcinoma with histiocytoid morphology. The carcinoma grows in rows or sheets of cells with abundant foamy to eosinophilic cytoplasm. C: Nuclei are enlarged with prominent nucleoli. D,E: The neoplastic cells show strong immunoreactivity for keratin AE1:3 (D) and weak, granular reactivity for GCDFP-15 (E). |
Fibrosis and chronic inflammation are often present around ducts and lobules involved by apocrine DCIS to a much greater degree than in benign apocrine lesions, especially when the DCIS has intermediate or high nuclear grade. Immunostains for myoepithelial markers and cytokeratin performed individually or in combination are usually helpful for detecting microinvasion in diagnostically difficult cases, or when apocrine DCIS involving a sclerosing lesion mimics invasive carcinoma.
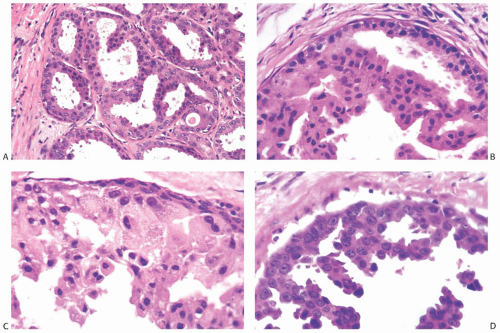 FIG. 19.7. Apocrine DCIS, micropapillary type. A,B: Low nuclear grade with slight nuclear pleomorphism and no nucleoli. C: Intermediate nuclear grade with moderate nuclear pleomorphism. The cells have variable amounts of cytoplasm. D: Micropapillary intraductal carcinoma with nucleoli in intermediate-grade nuclei. |
The absence of inmmunoreactive myoepithelium in an apocrine lesion devoid of cytologic atypia is not an absolute criterion for the diagnosis of carcinoma. Histologically and cytologically benign cystic and papillary apocrine lesions with little or no detectable myoepithelium have been described by few investigators.41,42,43 Cserni41 described two apocrine papillary lesions with no detectable myoepithelium, and Tramm et al.43 found large gaps in p63-positive myoepithelium surrounding apocrine metaplasia, especially papillary lesions, in the absence of cytologic atypia (Fig. 19.13). Immunoreactivity for calponin was more reliable for detecting myoepithelial cells in apocrine metaplasia, although some lesions were calponin-negative and p63-positive. Immunoreactive myoepithelium, however, is usually detectable in ducts involved by apocrine DCIS.
Despite using a panel of multiple myoepithelial markers, Seal et al.44 were unable to detect immunoreactive myoepithelium in the fibrovascular cores and at the periphery of five apocrine papillary tumors ranging in size from 1.2 to 4.5 cm. These findings and the presence of cytologic atypia and/or mitotic activity led the authors to classify the lesions as encapsulated papillary variants of apocrine carcinoma.
Awareness of these findings is important, as they could potentially be misleading in some cases. When evaluating an apocrine lesion, and especially a papillary apocrine lesion, it is critical to determine whether the apocrine epithelial proliferation shows cytologic and architectural atypia, and these parameters need to guide the diagnostic interpretation. In addition, when ruling out invasive carcinoma, it is important to use a panel of myoepithelial markers.
Atypical Apocrine Lesions
Atypical apocrine proliferations often occur in sclerosing lesions, such as papilloma, radial scar, and SA (Figs. 19.14 and 19.15). The cytomorphology of the atypical apocrine cells is similar to that of low-grade or intermediate-grade apocrine DCIS, but the proliferation lacks evidence of expansive growth, complex architecture, and/or necrosis that would qualify the lesion as DCIS. An important consideration in establishing a diagnosis of apocrine DCIS is the determination that the lesion has a structural growth pattern customarily associated with a nonapocrine form of DCIS. This is
a particularly significant factor when evaluating cytologically atypical apocrine components in lesions such as SA or radial scar. Carter and Rosen45 described sclerosing breast lesions with atypical apocrine epithelium, which were characterized by nuclear atypia, varying degrees of cytoplasmic clearing, and rare mitoses. The distinction from DCIS was based in most cases on the absence of sufficient epithelial proliferation to produce the characteristic growth patterns of intraductal carcinoma. Seidman et al.46 subsequently used similar criteria to define atypical apocrine adenosis. Rarely, the cytologic features are so abnormal in such lesions that a diagnosis of carcinoma in situ is indicated in the absence of a characteristic in situ carcinomatous structure.
a particularly significant factor when evaluating cytologically atypical apocrine components in lesions such as SA or radial scar. Carter and Rosen45 described sclerosing breast lesions with atypical apocrine epithelium, which were characterized by nuclear atypia, varying degrees of cytoplasmic clearing, and rare mitoses. The distinction from DCIS was based in most cases on the absence of sufficient epithelial proliferation to produce the characteristic growth patterns of intraductal carcinoma. Seidman et al.46 subsequently used similar criteria to define atypical apocrine adenosis. Rarely, the cytologic features are so abnormal in such lesions that a diagnosis of carcinoma in situ is indicated in the absence of a characteristic in situ carcinomatous structure.
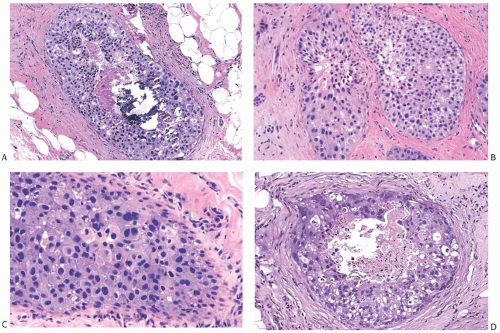 FIG. 19.8. Apocrine DCIS, solid type. A: Central necrosis, calcification, and intermediate nuclear grade. B: Low and intermediate nuclear grade. C: Low to high nuclear grade. D: Central necrosis, calcification, and pleomorphic nuclei. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree