http://evolve.elsevier.com/Edmunds/NP/
The pandemic of HIV has claimed millions of lives worldwide. The recommended use of antiretroviral agents in clinical practice will continue to evolve as new information from clinical trials and research becomes available. Infectious disease specialists generally provide treatment for patients with HIV infection because of the difficulty involved in keeping current with the latest treatment protocols. Primary care providers generally are concerned with prevention of transmission of the virus. Yet, having knowledge of current drug therapies and their side effects is necessary because HIV-infected patients rely on their primary care provider to help them evaluate their complaints and to provide treatment to some of them even to a limited extent.
Therapeutic Overview of HIV and Retroviruses
HIV is a retrovirus from the family of viruses referred to as lentiviruses. Retroviruses are viruses that replicate through the use of the reverse transcriptase enzyme, which allows the virus to incorporate its genome into that of certain host cells. This key enzyme transcripts the RNA into double-stranded DNA, resulting in insertion of the viruses’ genomes into CD4 receptor–containing cells. The primary CD4 receptor–containing cells are tissue-based macrophages and helper T-cells. Three primary categories of human retroviruses have been identified: T-cell leukemia retroviruses, endogenous viruses, and human immunodeficiency viruses (HIV-1 and HIV-2). This chapter discusses in detail only HIV-1.
Pathophysiology
The HIV virus attaches to the CD4 protein with the help of co-receptors (CXCR4 or CCR5) found on T-helper lymphocytes and other cells such as macrophages and dendritic cells. The HIV virus then fuses its membrane with that of the host cell and inserts its genetic material into the cytoplasm. The viral genetic material then is transcribed into double-stranded DNA called proviral DNA (Figure 68-1). The HIV enzyme, reverse transcriptase, is responsible for creating double-stranded DNA from viral RNA. Once produced, this DNA often becomes integrated into the chromosomal DNA of the host cell. The HIV DNA is expressed by the host cell’s genetic machinery. Expression of HIV DNA creates new HIV RNA genetic material and messenger RNA. The messenger RNA codes for the development of HIV polyproteins that must be cleaved, or separated, into individual proteins by the HIV enzyme protease if infectious virions are to be produced. Once this occurs, new virions are assembled and bud from the host cell’s membrane; they are able to infect new cells.
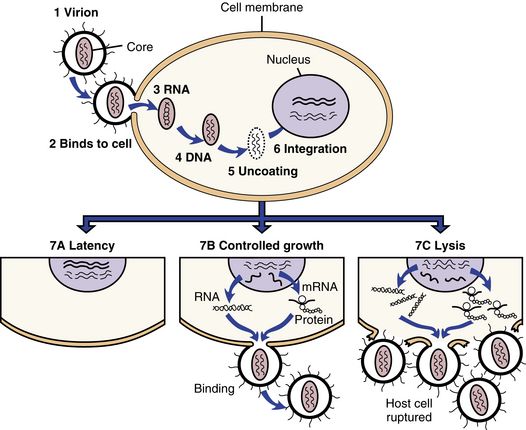
FIGURE 68-1 Infection and cellular outcomes of HIV.
HIV infection begins (1) when a virion, or virus particle, (2) binds to the outside of a susceptible cell and fuses with it, (3) injecting the core proteins and two strands of viral RNA. Uncoating occurs, during which the core proteins are removed and viral RNA is released into the cytoplasm of the infected cell. (4) The double-stranded DNA (provirus) migrates to the nucleus, (5) uncoats itself, and (6) is integrated into the cell’s own DNA. The provirus then can do a couple of things; it can (7A) remain latent or (7B) activate cellular mechanisms to copy its genes into RNA, some of which is translated into viral proteins or ribosomes. The proteins and additional RNA then are assembled into new virions that bud from the cell. The process can take place slowly while sparing the host cell (7B), or so rapidly that the cell is lysed or ruptured (7C). (From Edmunds MW: Introduction to Clinical Pharmacology, ed 7, St. Louis, 2013, Mosby.)
Disease Process
The defining stages of HIV infection through progression to AIDS have changed over the years as drug treatments have become extremely effective in preventing the opportunistic infections that once led to severe debilitation and death. The disease starts with a primary infection and then progresses to early, middle, and advanced or late-stage HIV infection or AIDS.
The disease seems to be divided into a primary phase, which includes initial acute infection that almost always presents with a mild to moderate viral syndrome that often mimics such infections as infectious mononucleosis. This stage includes the development of antibody production and the stabilization of viral load levels. It is at this time that the patient is most infectious. Early and middle stages of HIV infection can be fairly asymptomatic and represent the time when the virus entrenches itself in the architecture of the host’s immune system. The virus during this time destroys normal lymphoid architecture and creates reservoirs that are difficult to eradicate despite the best drug treatment. CD4 and CD8 cells undergo immunologic changes that render them useless in effectively killing and/or controlling HIV infection, leading to rapid HIV replication and mutation. It is during this stage that CD4 counts decrease dangerously to 200 to 300 cells/mm3.
Advanced or late stages of HIV infection show continued falling of CD4 counts, with drops to 50 cells/mm3. The patient experiences neurologic changes that are heralded by dementia, peripheral neuropathy, and myelopathy. Further immune system collapse occurs with the onset of opportunistic infections such as Pneumocystis jiroveci pneumonia, Toxoplasmosis, Mycobacterium avium complex, and multiple viral primary or reactivated infections such as herpes simplex or varicella-zoster. Chronic illness leads to constitutional disease with muscle wasting, weight loss, fevers, and severe fatigue. Malignancy with Kaposi’s sarcoma is also seen. The compilation of AIDS and the sequelae of opportunistic infections results finally in death.
Assessment
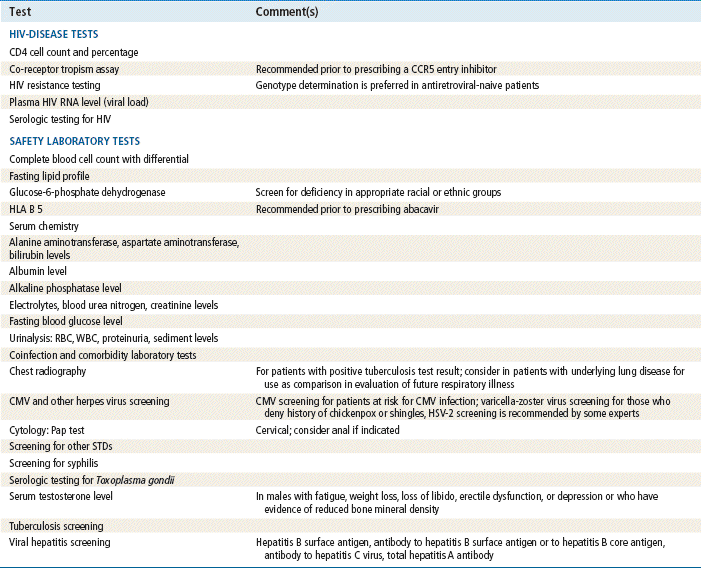
More than a quarter of all new HIV cases in the US in 2010 involved 13- to 24-year-olds. Sixty percent of these young people didn’t even know they were infected. Thus, testing must be started at earlier age if the spread is to be controlled and treatment initiated as quickly as possible. The following baseline information should be obtained before a patient is started on antiretrovirals:
See Table 68-1 for a comprehensive list of recommended laboratory studies for patients presenting with HIV per the 2009 Infectious Diseases Society of America.
Mechanism of Action
In the two decades since zidovudine (formerly known as AZT) was introduced, 24 additional agents in five drug classes have been approved; potent combination therapy has become a worldwide standard of care; morbidity and mortality in the developed world have been substantially reduced; and major antiretroviral regimens have been initiated throughout the developing world. Balanced against the progress that has been made is the identification of a surprising number of major toxic effects and recognition of drug class cross-resistance and the restrictions this places on alternate treatment regimens in the setting of treatment failure. Strict adherence is essential to prescribed therapy because nonadherence allows virus(es) to replicate to resistant strains.
Antiretroviral agents act to stop the production of new retroviruses by interfering with the ability of the retrovirus to replicate (see Figure 68-1). NRTIs disrupt replication of the virus at the point at which the virion is replicating its RNA to make DNA via the reverse transcriptase, the enzyme that copies viral RNA into DNA. NNRTIs resemble false nucleotides by binding within a mechanism to inhibit the reverse transcriptase enzyme activity. PIs prevent the protease enzyme from cleaving essential proteins into the HIV virion (Figure 68-2). Fusion inhibitors prevent HIV from entering target cells. Integrase strand transfer inhibitors (INSTIs) interfere with the process of DNA strand transfer from the viral genome to the host genome.
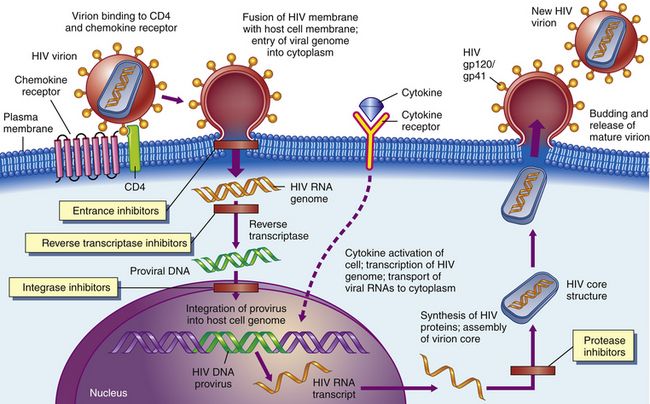
FIGURE 68-2 HIV is subject to attack by drugs at several stages.
Certain agents could block the binding of HIV to CD4 receptors on the surfaces of helper T-cells (1). Other agents might keep viral RNA and reverse transcriptase from leaving their protein coat (2). Drugs such as AZT and other dideoxynucleosides prevent the reverse transcription of viral RNA into viral DNA (3). Later, antisense oligonucleotides could block the translation of mRNA into viral proteins (4). Certain compounds could interfere with viral assembly by modifying such processes (5), and finally, antiviral agents such as interferon could keep the virus from assembling itself and budding out of the cell (6). (From McCance KL, Huether SE: Pathophysiology: The Biologic Basis for Disease in Adults and Children, ed 6, St. Louis. 2010. Mosby.)
Highly Active Antiretroviral Treatment
The great progress that has been demonstrated in the treatment of HIV has been made through the use of different drug combinations or drug cocktails or highly active antiretroviral treatment (HAART). HAART therapy is used to describe the combination of multiple antiretrovirals to achieve the maximum effect in viral load suppression to a goal of undetectable viral load levels. When HAART treatment is given, the following management strategy is recommended when therapy must be changed:
Genotypic or phenotypic testing is available as an in-vitro tool to examine resistance of HIV to antiretroviral agents. Genotypic assays detect drug resistance mutations that are present in reverse transcriptase, protease, and integrase genes. Phenotypic assays measure the ability of the virus to replicate within concentrations of the antiretrovirals. These assays facilitate selection of antiretroviral agents when drug regimens are changed. It is best to consult with an expert who can assist with interpretation of these results. The U.S. Department of Health and Human Services (DHHS) antiretroviral guidelines are a comprehensive reference for changing medications.
Individual drugs included in HAART therapy are discussed in the following paragraphs.
Reverse Transcriptase Inhibitors
Reverse transcriptase inhibitors prevent the HIV enzyme reverse transcriptase from creating HIV proviral DNA from viral RNA. This in turn prevents production of new viruses. Two primary categories of reverse transcriptase inhibitors have been identified: nucleoside reverse transcriptase inhibitors (NRTIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs).
NNRTIs must be phosphorylated within target cells to their active triphosphate form. It is important to note that the nucleotide analog reverse transcriptase inhibitor, tenofovir, is included in the same category as a nucleoside subclass. However, the structural difference between nucleotides and nucleosides is that the nucleotides already have a phosphate group, so only two steps of phosphorylation are required instead of three. Once these medications are in the active triphosphate form, they work through at least two mechanisms: chain termination and competitive inhibition.
Chain termination occurs when reverse transcriptase adds the reverse transcriptase inhibitor into the growing chain of HIV proviral DNA. Antiretroviral nucleoside analogs all have a modification in their sugar ring that prevents additional nucleotides (the building blocks of DNA) from being added. This stops the production of HIV proviral DNA, thereby preventing the formation of new HIV viruses.
Competitive inhibition of the endogenous nucleoside-5’-triphosphates is a process by which the phosphorylated reverse transcriptase inhibitor competes with and replaces the endogenous nucleoside-5’-triphosphates. The endogenous nucleoside-5’-triphosphates are necessary for the production of HIV proviral DNA. By competing with and replacing the nucleoside-5’-triphosphates, neither HIV proviral DNA nor new HIV viruses are produced.
NNRTIs do not require phosphorylation or intracellular processing to be activated. They are noncompetitive, binding to reverse transcriptase and inhibiting the function of this enzyme by binding at sites distinct from nucleoside binding sites.
Protease Inhibitors
One of the final stages of the HIV life cycle is the production of HIV polyproteins, for which coding is provided by the viral messenger RNA. These polyproteins must be cleaved or separated into individual proteins by the HIV enzyme known as protease if infectious virions are to be produced. Protease inhibitors block the HIV enzyme protease and thereby prevent certain HIV polyproteins from being cleaved or separated into the individual proteins necessary for the production of new infectious virions. This causes production of noninfectious HIV virions.
Fusion/Entry Inhibitors
Enfuvirtide is in a class of medications called fusion/entry inhibitors that work by preventing the AIDS virus from invading the white blood cells that are the primary targets of HIV. These inhibitors work by attaching themselves to proteins on the surfaces of T-cells or proteins on the surfaces of HIV cells. For HIV to bind to T-cells, the proteins on the outer coat of the HIV must bind to the surface receptors on T-cells. Fusion inhibitors prevent this from happening. Some fusion inhibitors target the gp120 or gp41 on the CD4 protein or, in the case of maraviroc, the CCR5 co-receptor. A profile test should be done prior to using maraviroc. If entry inhibitors are successful, HIV is unable to bind to the surfaces of T-cells and to gain entry into the cells.
HIV Integrase Inhibitors
The first of a new pharmacologic class of antiretroviral agents was approved in 2009. Accelerated approval was given for raltegravir tablets (400 mg) used in combination with other antiretroviral agents for the treatment of HIV-1 infection.
New products are constantly under development, and extensive research is in progress. A good deal of experimentation on effective drug combinations has been conducted. It will be essential for any clinician who treats HIV+ patients to consult the latest sources for prescribing and treatment information. One new 4-combination product, Stribild, is composed of a new drug, elvitegravir, and a new booster that allows it to be given once a day. It is combined with emtricitabine and tenofovir and is intended for patients who have never been treated for HIV infection.
Treatment Principles
Evidence-Based Recommendations
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


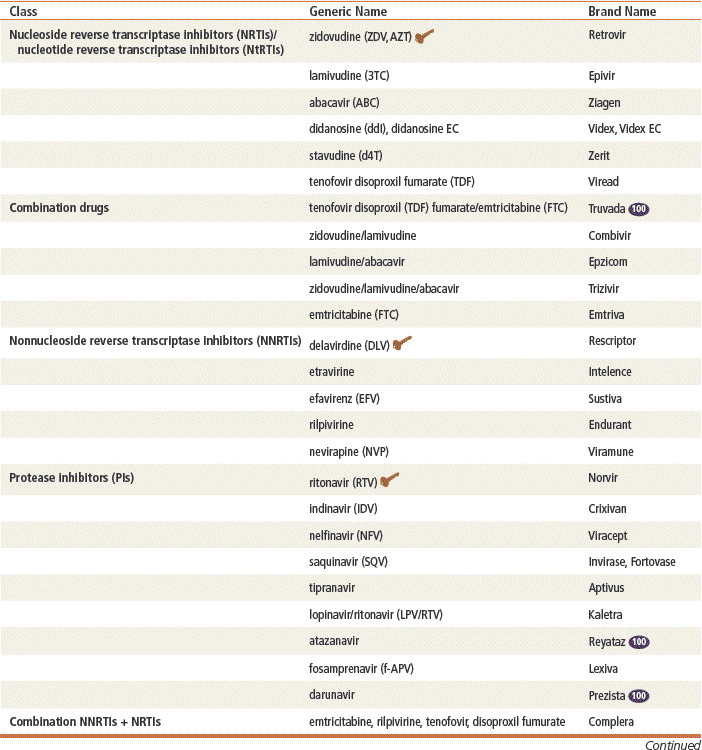

 Top 100 drug;
Top 100 drug;  key drug.
key drug.