Chapter 68 The HIV virus attaches to the CD4 protein with the help of co-receptors (CXCR4 or CCR5) found on T-helper lymphocytes and other cells such as macrophages and dendritic cells. The HIV virus then fuses its membrane with that of the host cell and inserts its genetic material into the cytoplasm. The viral genetic material then is transcribed into double-stranded DNA called proviral DNA (Figure 68-1). The HIV enzyme, reverse transcriptase, is responsible for creating double-stranded DNA from viral RNA. Once produced, this DNA often becomes integrated into the chromosomal DNA of the host cell. The HIV DNA is expressed by the host cell’s genetic machinery. Expression of HIV DNA creates new HIV RNA genetic material and messenger RNA. The messenger RNA codes for the development of HIV polyproteins that must be cleaved, or separated, into individual proteins by the HIV enzyme protease if infectious virions are to be produced. Once this occurs, new virions are assembled and bud from the host cell’s membrane; they are able to infect new cells. • Documentation of all medications and dosages • Past history and current medical problems, including depression, alcohol, and tobacco and substance use history • CD4 and plasma HIV RNA measurement. These studies help to assess a patient’s immunologic status and severity of infection. They also provide a means of measuring the efficacy of therapy. • HIV resistance testing (genotype). This test is used to identify transmitted drug-resistant viruses, as well as acquired resistance. This information helps with constructing a highly active antiretroviral treatment (HAART) regimen. • CBC; chemistry profile; LFTs; BUN and creatinine; UA; serologies for hepatitis A, B, and C; FLP; and fasting blood glucose See Table 68-1 for a comprehensive list of recommended laboratory studies for patients presenting with HIV per the 2009 Infectious Diseases Society of America. TABLE 68-1 Baseline and Screening Laboratory Studies for HIV/AIDS Recommendations from 2009 Infectious Diseases Society of America. Antiretroviral agents act to stop the production of new retroviruses by interfering with the ability of the retrovirus to replicate (see Figure 68-1). NRTIs disrupt replication of the virus at the point at which the virion is replicating its RNA to make DNA via the reverse transcriptase, the enzyme that copies viral RNA into DNA. NNRTIs resemble false nucleotides by binding within a mechanism to inhibit the reverse transcriptase enzyme activity. PIs prevent the protease enzyme from cleaving essential proteins into the HIV virion (Figure 68-2). Fusion inhibitors prevent HIV from entering target cells. Integrase strand transfer inhibitors (INSTIs) interfere with the process of DNA strand transfer from the viral genome to the host genome. 1. Base changes on drug regimen on the following: • CD4 decline measured on two occasions • Virologic failure demonstrated by increases in HIV RNA viral load 2. Perform genotypic or phenotypic resistance testing while the patient is still on the old regimen, and use the results to help choose a new regimen. If the HIV RNA is <500-1000 copies/ml, amplification of virus for resistance testing may not always be successful. 3. Always change at least two of the antiretrovirals in the regimen. 4. Avoid choosing agents with resistance patterns that overlap those the patient has failed. 5. Avoid choosing agents with side effects similar to those to which the patient is intolerant. Individual drugs included in HAART therapy are discussed in the following paragraphs. • Panel on Antiretroviral Guidelines for Adults and Adolescents: Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services. October 14, 2011; 1-167 (updated March 27, 2012). • Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children: Guidelines for the use of antiretroviral agents in pediatric HIV infection. Department of Health and Human Services. August 11, 2011; 1-268. Available at http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pd. • Panel on Treatment of HIV-Infected Pregnant Women and Prevention of Perinatal Transmission: Recommendations for use of antiretroviral drugs in pregnant HIV-1-infected women for maternal health and interventions to reduce perinatal HIV transmission in the United States. Department of Health and Human Services. September 14, 2011; 1-207. • Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 update by the HIV Medicine Association of the Infectious Disease Society of America. September 1, 2009; 651-681.
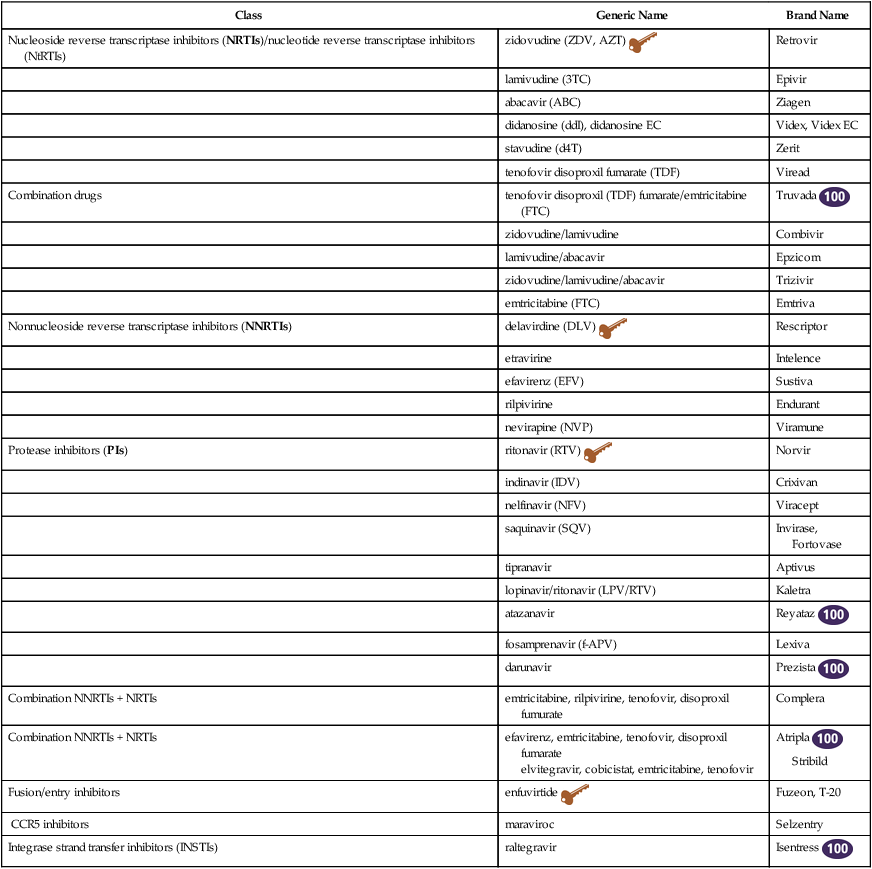
Antiretroviral Medications
Class
Generic Name
Brand Name
Nucleoside reverse transcriptase inhibitors (NRTIs)/nucleotide reverse transcriptase inhibitors (NtRTIs)
zidovudine (ZDV, AZT) ![]()
Retrovir
lamivudine (3TC)
Epivir
abacavir (ABC)
Ziagen
didanosine (ddI), didanosine EC
Videx, Videx EC
stavudine (d4T)
Zerit
tenofovir disoproxil fumarate (TDF)
Viread
Combination drugs
tenofovir disoproxil (TDF) fumarate/emtricitabine (FTC)
Truvada ![]()
zidovudine/lamivudine
Combivir
lamivudine/abacavir
Epzicom
zidovudine/lamivudine/abacavir
Trizivir
emtricitabine (FTC)
Emtriva
Nonnucleoside reverse transcriptase inhibitors (NNRTIs)
delavirdine (DLV) ![]()
Rescriptor
etravirine
Intelence
efavirenz (EFV)
Sustiva
rilpivirine
Endurant
nevirapine (NVP)
Viramune
Protease inhibitors (PIs)
ritonavir (RTV) ![]()
Norvir
indinavir (IDV)
Crixivan
nelfinavir (NFV)
Viracept
saquinavir (SQV)
Invirase, Fortovase
tipranavir
Aptivus
lopinavir/ritonavir (LPV/RTV)
Kaletra
atazanavir
Reyataz ![]()
fosamprenavir (f-APV)
Lexiva
darunavir
Prezista ![]()
Combination NNRTIs + NRTIs
emtricitabine, rilpivirine, tenofovir, disoproxil fumurate
Complera
Combination NNRTIs + NRTIs
efavirenz, emtricitabine, tenofovir, disoproxil fumarate
elvitegravir, cobicistat, emtricitabine, tenofovir
Atripla ![]()
Stribild
Fusion/entry inhibitors
enfuvirtide ![]()
Fuzeon, T-20
CCR5 inhibitors
maraviroc
Selzentry
Integrase strand transfer inhibitors (INSTIs)
raltegravir
Isentress ![]()
Therapeutic Overview of HIV and Retroviruses
Anatomy and Physiology
Pathophysiology
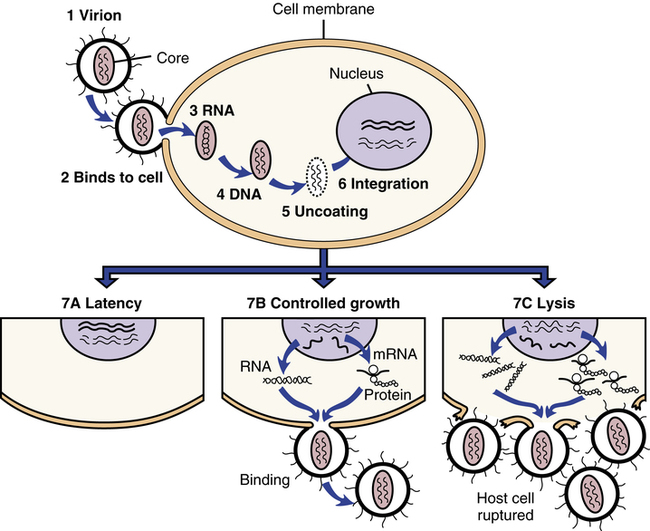
HIV infection begins (1) when a virion, or virus particle, (2) binds to the outside of a susceptible cell and fuses with it, (3) injecting the core proteins and two strands of viral RNA. Uncoating occurs, during which the core proteins are removed and viral RNA is released into the cytoplasm of the infected cell. (4) The double-stranded DNA (provirus) migrates to the nucleus, (5) uncoats itself, and (6) is integrated into the cell’s own DNA. The provirus then can do a couple of things; it can (7A) remain latent or (7B) activate cellular mechanisms to copy its genes into RNA, some of which is translated into viral proteins or ribosomes. The proteins and additional RNA then are assembled into new virions that bud from the cell. The process can take place slowly while sparing the host cell (7B), or so rapidly that the cell is lysed or ruptured (7C). (From Edmunds MW: Introduction to Clinical Pharmacology, ed 7, St. Louis, 2013, Mosby.)
Assessment
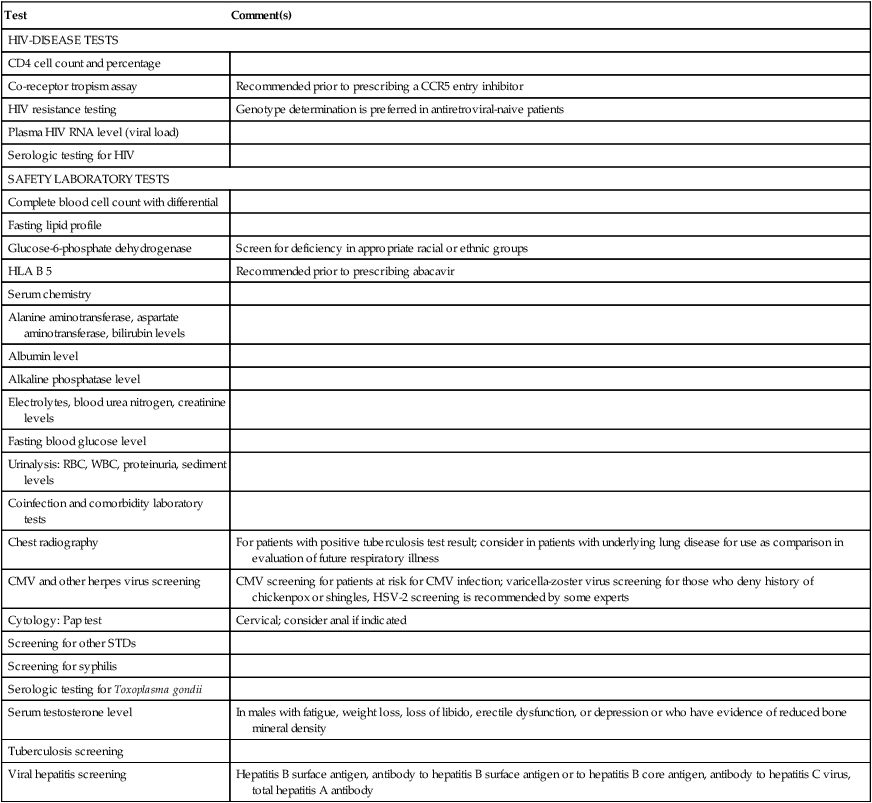
Test
Comment(s)
HIV-DISEASE TESTS
CD4 cell count and percentage
Co-receptor tropism assay
Recommended prior to prescribing a CCR5 entry inhibitor
HIV resistance testing
Genotype determination is preferred in antiretroviral-naive patients
Plasma HIV RNA level (viral load)
Serologic testing for HIV
SAFETY LABORATORY TESTS
Complete blood cell count with differential
Fasting lipid profile
Glucose-6-phosphate dehydrogenase
Screen for deficiency in appropriate racial or ethnic groups
HLA B 5
Recommended prior to prescribing abacavir
Serum chemistry
Alanine aminotransferase, aspartate aminotransferase, bilirubin levels
Albumin level
Alkaline phosphatase level
Electrolytes, blood urea nitrogen, creatinine levels
Fasting blood glucose level
Urinalysis: RBC, WBC, proteinuria, sediment levels
Coinfection and comorbidity laboratory tests
Chest radiography
For patients with positive tuberculosis test result; consider in patients with underlying lung disease for use as comparison in evaluation of future respiratory illness
CMV and other herpes virus screening
CMV screening for patients at risk for CMV infection; varicella-zoster virus screening for those who deny history of chickenpox or shingles, HSV-2 screening is recommended by some experts
Cytology: Pap test
Cervical; consider anal if indicated
Screening for other STDs
Screening for syphilis
Serologic testing for Toxoplasma gondii
Serum testosterone level
In males with fatigue, weight loss, loss of libido, erectile dysfunction, or depression or who have evidence of reduced bone mineral density
Tuberculosis screening
Viral hepatitis screening
Hepatitis B surface antigen, antibody to hepatitis B surface antigen or to hepatitis B core antigen, antibody to hepatitis C virus, total hepatitis A antibody
Mechanism of Action
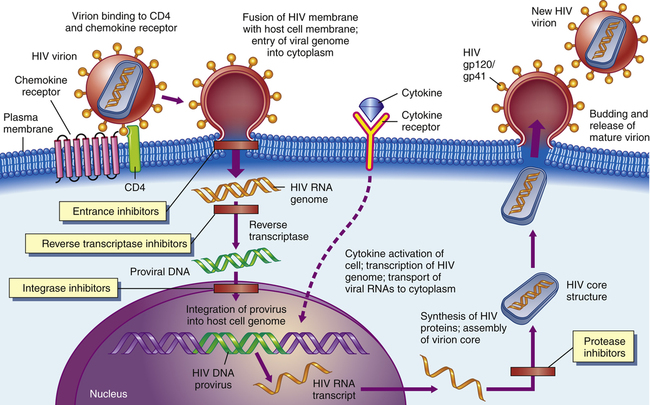
Certain agents could block the binding of HIV to CD4 receptors on the surfaces of helper T-cells (1). Other agents might keep viral RNA and reverse transcriptase from leaving their protein coat (2). Drugs such as AZT and other dideoxynucleosides prevent the reverse transcription of viral RNA into viral DNA (3). Later, antisense oligonucleotides could block the translation of mRNA into viral proteins (4). Certain compounds could interfere with viral assembly by modifying such processes (5), and finally, antiviral agents such as interferon could keep the virus from assembling itself and budding out of the cell (6). (From McCance KL, Huether SE: Pathophysiology: The Biologic Basis for Disease in Adults and Children, ed 6, St. Louis. 2010. Mosby.)
Highly Active Antiretroviral Treatment
Treatment Principles
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


