II. Antimicrobial Prophylaxis for Surgical Procedures
A. The use of antimicrobial prophylaxis in surgery involves a risk-to-benefit evaluation, which varies depending on the nature of the operative procedure (Table 41-2). It is recommended that prophylactic antimicrobials should be administered intravenously (IV) within 1 hour of surgical incision (tissue concentration of the antibiotic should exceed the minimum inhibitory concentration [MIC] associated with the procedure and or patient characteristics from the time of incision to the completion of surgery). Antibiotic treatment is not recommended for longer than 24 hours.
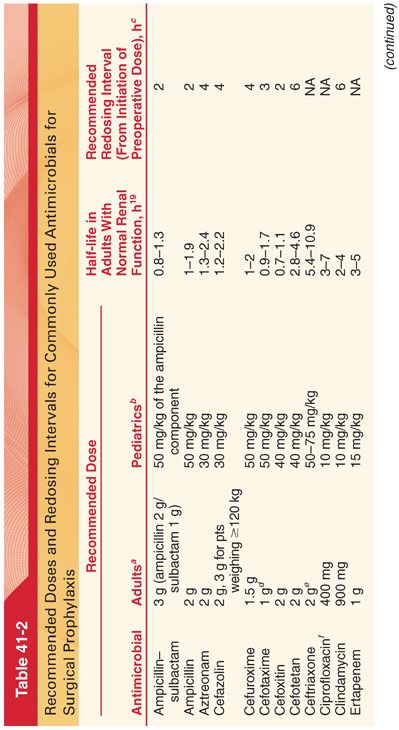
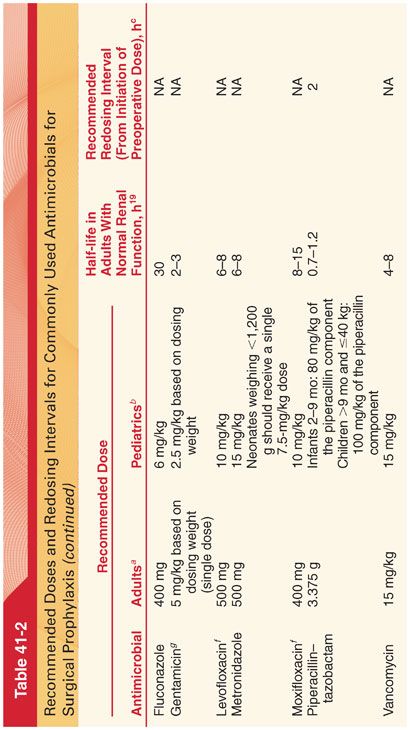

B. Because of their wide therapeutic index and low incidence of side effects, cephalosporins (most often a cost-effective first-generation cephalosporin such as cefazolin) are the antimicrobials of choice for surgical procedures in which skin flora and normal flora of the gastrointestinal and genitourinary tracts are the most likely pathogens.
1. Cephalosporins can safely be used in patients with an allergic reaction to penicillins that is not an immunoglobulin E (IgE)–mediated reaction (anaphylaxis, urticaria, bronchospasm) or exfoliative dermatitis (Stevens-Johnson syndrome).
2. In patients with documented IgE-mediated anaphylactic reactions, β-lactam antibiotics can usually be substituted with clindamycin or vancomycin.
3. Routine prophylaxis with vancomycin is not recommended for any patient population in the absence of documented or highly suspected colonization or infection with methicillin-resistant Staphylococcus aureus (recent hospitalization or nursing home stay and hemodialysis patients) or known IgE-mediated response to β-lactam antibiotics.
C. Pseudomembranous colitis is the most frequent complication of prophylactic antimicrobials, including the IV cephalosporins (Table 41-3).
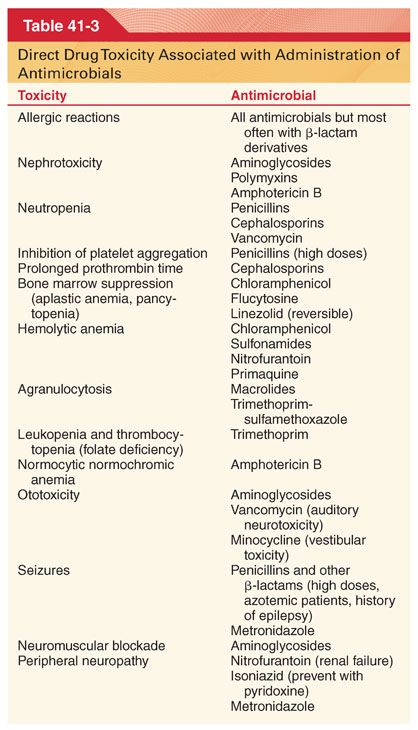
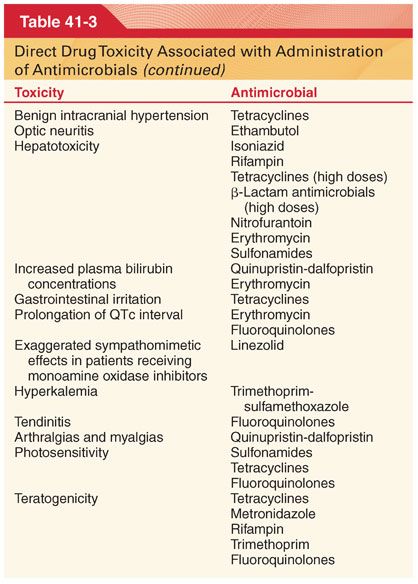
III. Antimicrobial Selection. Prompt identification of the causative organism is essential for the selection of appropriate antimicrobial drugs to treat ongoing infection. Infections behind obstructing lesions such as pneumonia behind a blocked bronchus will not respond to antimicrobials until the obstruction is relieved.
A. Nosocomial Infections
1. Nearly 80% of nosocomial infections occur in three sites (urinary tract, respiratory system, and bloodstream). The incidence of nosocomial infections is highly associated with the use of devices such as ventilators, vascular access catheters, and urinary catheters. Intravascular access catheters are the most common cause of bacteremia or fungemia in hospitalized patients.
2. The organism infecting access catheters most commonly comes from the colonized hub or lumen and reflect skin flora (S. aureus and Staphylococcus epidermidis). Initial therapy of suspected intravascular catheter infection usually includes vancomycin because of the high incidence of methicillin-resistant S. aureus and S. epidermidis in the nosocomial environment.
IV. Special Patient Groups
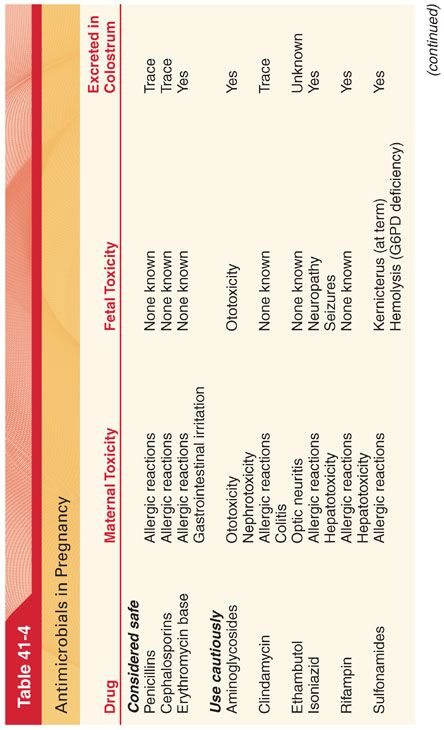
A. Parturients (Table 41-4). Most antimicrobials cross the placenta and enter maternal milk. The immature fetal liver may lack enzymes necessary to metabolize certain drugs such that pharmacokinetics and toxicities in the fetus are often different from those in older children and adults. Teratogenicity is a concern when any drug is administered during early pregnancy.

B. Elderly Patients
1. Physiologic changes that occur with increasing age can alter oral absorption, distribution metabolism, and excretion of antimicrobials.
2. Penicillins and cephalosporins, because of their large therapeutic index, obviate the need for significant changes in dosage schedules in elderly patients who have normal serum creatinine concentrations.
3. Administration of aminoglycosides and vancomycin to elderly patients may require adjustments in dosing regimens.
C. HIV-Infected Patients. There has been concern about increased risk of postoperative infection in HIV-infected patients based on their increased risk for opportunistic infection in the setting of reduced T4 cell counts (goal is preoperative control on an antiretroviral regimen with preserved T4 cell counts).
V. Antibacterial Drugs Commonly Used in the Perioperative Period
A. Penicillins. The bactericidal action of penicillins reflects the ability of these antimicrobials to interfere with the synthesis of peptidoglycan, which is an essential component of cell walls of susceptible bacteria. Cell membranes of resistant gram-negative bacteria are in general resistant to penicillins because they prevent access to sites where synthesis of peptidoglycan is taking place.
1. Clinical Indications
a. Penicillin is the drug of choice for treatment of pneumococcal, streptococcal, and meningococcal infections. Penicillin is the drug of choice for treating all forms of actinomycosis and clostridial infections causing gas gangrene.
b. Prophylactic administration of penicillin is highly effective against streptococcal infections, accounting for its value in patients with rheumatic fever.
c. Transient bacteremia occurs in the majority of patients undergoing dental extractions, emphasizing the importance of prophylactic penicillin in patients with congenital or acquired heart disease or tissue implants undergoing dental procedures.
d. Intrathecal administration of penicillins is not recommended because these drugs are potent convulsants when administered by this route.
2. Excretion. Renal excretion of penicillin is rapid (60% to 90% of an intramuscular [IM] dose is excreted in the first hour), such that the plasma concentration decreases to 50% of its peak value within 1 hour after injection.
3. Duration of Action
a. Methods to prolong the duration of action of penicillin include the simultaneous administration of probenecid, which blocks the renal tubular secretion of penicillin.
b. Procaine penicillin contains 120 mg of the local anesthetic for every 300,000 units of the antimicrobial (hypersensitivity to procaine must be considered).
B. Penicillinase-Resistant Penicillins
1. The major mechanism of resistance to the penicillins is bacterial production of β-lactamase enzymes that hydrolyze the β-lactam ring, rendering the antimicrobial molecule inactive (methicillin, oxacillin, nafcillin, cloxacillin, and dicloxacillin are not susceptible to hydrolysis by staphylococcal penicillinases).
2. Penetration of nafcillin into the central nervous system (CNS) is sufficient to treat staphylococcal meningitis.
C. Penicillinase-Susceptible Broad-Spectrum Penicillins (Second-Generation Penicillins). Broad-spectrum penicillins have a wider range of activity than other penicillins, being bactericidal against gram-positive and gram-negative bacteria. They are all inactivated by penicillinase produced by certain gram-negative and gram-positive bacteria. Therefore, these drugs are not effective against most staphylococcal infections.
1. Ampicillin (α-aminobenzylpenicillin) has a broader range of activity than penicillin G. Its spectrum encompasses not only pneumococci, meningococci, gonococci, and various streptococci but also a number of gram-negative bacilli, such as Haemophilus influenzae and Escherichia coli.
a. Approximately 50% of an oral dose of ampicillin is excreted unchanged by the kidneys in the first 6 hours, emphasizing that renal function greatly influences the duration of action of this antimicrobial.
b. Among the penicillins, ampicillin is associated with the highest incidence of skin rash (9%), which typically appears 7 to 10 days after initiation of therapy (often due to protein impurities in the commercial preparation of the drug and do not represent true allergic reactions).
2. Amoxicillin. Its spectrum of activity is identical to that of ampicillin, but it is more efficiently absorbed from the gastrointestinal tract than ampicillin, and effective concentrations are present in the circulation for twice as long.
D. Extended-Spectrum Carboxypenicillins (Third-Generation Penicillins)
1. Carbenicillin
a. The principal advantage of carbenicillin is its effectiveness in the treatment of infections caused by Pseudomonas aeruginosa and certain Proteus strains that are resistant to ampicillin.
b. This antimicrobial is penicillinase susceptible and therefore ineffective against most strains of S. aureus. Carbenicillin is not absorbed from the gastrointestinal tract; therefore, it must be administered parenterally.
c. Approximately 85% of the unchanged drug is recovered in urine over 9 hours. Probenecid, by delaying renal excretion of the drug, increases the plasma concentration of carbenicillin by approximately 50%.
d. The sodium load administered with a large dose of carbenicillin (30 to 40 g) is considerable because greater than 10% of carbenicillin is sodium (about 5 mEq/g). Congestive heart failure may develop in susceptible patients in response to this acute drug-produced sodium load.
e. Hypokalemia and metabolic alkalosis may occur because of obligatory excretion of potassium with the large amount of nonreabsorbable carbenicillin.
f. Carbenicillin interferes with normal platelet aggregation such that bleeding time is prolonged but platelet count remains normal.
E. Extended-spectrum acylaminopenicillins (fourth-generation penicillins) have the broadest spectrum of activity of all the penicillins. Like the carboxypenicillins, the acylaminopenicillins are derivatives of ampicillin. These drugs are ineffective against penicillinase-producing strains of S. aureus.
F. Penicillin β-Lactamase Inhibitor Combinations. Clavulanic acid, sulbactam, and tazobactam are β-lactam compounds that bind irreversibly to the β-lactamase enzymes that are produced by many bacteria, thus inactivating these enzymes and rendering the organisms sensitive to β-lactamase–susceptible penicillins.
VI. Cephalosporins, like the penicillins, are bactericidal antimicrobials that inhibit bacterial cell wall synthesis and have a low intrinsic toxicity. Bacteria can produce cephalosporinases (β-lactamases) that disrupt the β-lactam structure of cephalosporins and thus inhibit their antimicrobial activity. Like the newer penicillins, the new cephalosporins have an extraordinarily broad spectrum of antimicrobial action. Nephrotoxicity owing to cephalosporins, with the exception of cephaloridine, is less frequent than after administration of aminoglycosides or polymyxins. The incidence of allergic reactions in patients being treated with cephalosporins ranges from 1% to 10%. The majority of the allergic reactions consist of cutaneous manifestations that occur 24 hours after drug exposure. Life-threatening anaphylaxis is estimated to occur in 0.02% of treated patients. Because the cephalosporins share immunologic cross-reactivity, patients who are allergic to one cephalosporin are likely to be allergic to others. The possibility of cross-reactivity between cephalosporins and penicillins seems to be very infrequent, and cephalosporins are often selected as alternative antimicrobials in patients with a history of penicillin allergy.
A. Cephalosporins and Allergy to Penicillins
1. Hypersensitivity is the most common adverse reaction to β-lactam antimicrobials. Allergic reactions are noted in 1% to 10% of patients treated with penicillins, making these antimicrobials the most allergenic of all drugs.
a. Most often, the allergic response is a delayed reaction characterized by a maculopapular rash and/or fever.
b. Less often but more serious is immediate hypersensitivity that is mediated by IgE antibodies. Manifestations of immediate hypersensitivity may include laryngeal edema, bronchospasm, and cardiovascular collapse.
c. Allergic reactions may occur in the absence of previous known exposure to any of the penicillins. This may reflect prior unrecognized exposure to penicillin, presumably in ingested foods.
d. Allergic reactions can occur with any dose or route of administration, although severe anaphylactic reactions are more often associated with parenteral than with oral administration.
2. Some patients who experience cutaneous reactions may continue to receive the offending penicillin or receive the same penicillin in the future without experiencing a similar response.
B. Cross-Reactivity. The presence of a common nucleus (β-lactam ring) in the structure of all penicillins means that allergy to one penicillin increases the likelihood of an allergic reaction to another penicillin (actual cross-reactivity is rare).
C. Classification. Cephalosporins are classified as first-, second-, and third-generation because of their antimicrobial spectrum. First-generation cephalosporins are inexpensive, exhibit low toxicity, and are as active as second- and third-generation cephalosporins against staphylococci and nonenterococcal streptococci (first-generation cephalosporins have been commonly selected for antimicrobial prophylaxis in patients undergoing cardiovascular, orthopedic, biliary, pelvic, and intraabdominal surgery). All cephalosporins can penetrate into joints and can readily cross the placenta.
1. First-Generation Cephalosporins
a. Cephalothin is the prototype of first-generation cephalosporins (excreted largely unaltered by the kidneys, emphasizing the need to decrease the dose in the presence of renal dysfunction). Oral absorption is poor and IM injection is painful, accounting for its common administration by the IV route. Although cephalothin is present in many tissues and fluids, it does not enter the cerebrospinal fluid in significant amounts and is not recommended for treatment of meningitis.
b. Cefazolin has essentially the same antimicrobial spectrum as cephalothin but has the advantage of achieving higher blood levels, presumably due to slower renal elimination (viewed as the drug of choice for antimicrobial prophylaxis for many surgeries). This drug is well tolerated after IM or IV injection.
2. Second-Generation Cephalosporins
a. Cefoxitin and cefamandole are examples of second-generation cephalosporins with extended activity against gram-negative bacteria.
b. Cefoxitin is resistant to cephalosporinases produced by gram-negative bacteria.
c. Cefamandole is pharmacologically similar to cefoxitin, but its methylthiotetrazole side chain poses a risk of bleeding and disulfiram-like reactions with concurrent use of alcohol.
d. Both drugs are excreted predominantly unchanged by the kidneys.
3. Third-Generation Cephalosporins
a. Third-generation cephalosporins have an enhanced ability to resist hydrolysis by the β-lactamases of many gram-negative bacilli.
b. Unlike older cephalosporins, the third-generation cephalosporins achieve therapeutic levels in the cerebrospinal fluid and can be used to treat meningitis.
c. Cefotaxime was the first third-generation cephalosporin and has been effective in a broad range of infections, including meningitis caused by gram-negative bacilli other than Pseudomonas.
d. Ceftriaxone has the longest elimination half-time of any third-generation cephalosporin and is highly effective against gram-negative bacilli.
e. The spectrum of activity of cefixime and a single daily dose make it attractive for upper respiratory tract infections, but less expensive alternatives are available.
VII. Other β-Lactam Antimicrobials
A. Aztreonam
1. The antimicrobial activity of this drug is limited to gram-negative bacteria. Aztreonam is not absorbed from the gastrointestinal tract, but therapeutic blood levels are achieved after IM or IV administration in most body tissues and fluids, including cerebrospinal fluid.
2. Clearance is principally by glomerular filtration.
3. Because aztreonam combines the activity of the aminoglycosides with the low toxicity of the β-lactam antimicrobials, it can replace aminoglycosides in the treatment of many gram-negative infections.
VIII. Aminoglycoside antimicrobials are poorly lipid-soluble antimicrobials (<1% of an orally administered aminoglycoside is absorbed) that are rapidly bactericidal for aerobic gram-negative bacteria. There is a linear relationship between the plasma creatinine concentration and the elimination half-time of aminoglycosides.
A. Streptomycin is rarely selected because of the rapid emergence of resistant organisms, the frequent occurrence of vestibular damage during prolonged treatment, and the availability of less toxic antimicrobials.
B. Gentamicin is active against P. aeruginosa as well as the gram-negative bacilli (penetrates pleural, ascitic, and synovial fluids in the presence of inflammation). Monitoring plasma concentrations of gentamicin is the best approach for recognizing potentially toxic levels (>9 μg/mL).
C. Amikacin is a semisynthetic derivative of kanamycin that has the advantage of not being associated with the development of resistance. The principal use of amikacin is in the treatment of infections caused by gentamicin- or tobramycin-resistant gram-negative bacilli. The incidence of nephrotoxicity and ototoxicity is similar to that produced by gentamicin.
D. Neomycin is commonly used for topical application to treat infections of the skin (as after burn injury), cornea, and mucous membranes. Allergic reactions occur in 6% to 8% of patients treated with topical neomycin. Oral neomycin does not undergo systemic absorption and is thus administered to decrease bacterial flora in the intestine before gastrointestinal surgery and as an adjunct to the therapy of hepatic coma (decreases blood ammonia concentrations).
E. Side Effects
1. Ototoxicity reflects drug-induced destruction of vestibular or cochlear sensory hairs that is dose-dependent and most likely occurs with chronic therapy, especially in elderly patients, in whom renal dysfunction is more likely. Furosemide, mannitol, and probably other diuretics seem to accentuate the ototoxic effects of aminoglycosides.
2. Nephrotoxicity
a. Aminoglycosides can produce acute tubular necrosis that initially manifests as an inability to concentrate urine and the appearance of proteinuria and red blood cell casts (usually reversible if the drug is discontinued).
b. Neomycin is the most nephrotoxic of the aminoglycosides and therefore is not administered by the parenteral route.
3. Skeletal muscle weakness is most likely because of the ability of aminoglycosides to inhibit the prejunctional release of acetylcholine while also decreasing postsynaptic sensitivity to the neurotransmitter (IV administration of calcium overcomes the effect of aminoglycosides at the neuromuscular junction).
a. Patients with myasthenia gravis are uniquely susceptible to skeletal muscle weakness if treated with an aminoglycoside.
b. Administration of a single dose of an aminoglycoside is unlikely to produce skeletal muscle weakness in an otherwise healthy patient.
IX. Macrolides are stable in the presence of acidic gastric fluid, and as a result, these antimicrobials are well absorbed from the gastrointestinal tract.
A. Erythromycin has a spectrum of activity that includes most gram-positive bacteria. In patients who cannot tolerate penicillins or cephalosporins, erythromycin or clindamycin are an effective alternative for the treatment of streptococcal pharyngitis, bronchitis, and pneumonia. Severe nausea and vomiting may accompany infusion of erythromycin.
1. Effects on QTc. Oral erythromycin prolongs cardiac repolarization and is associated with reports of torsades de pointes.
B. Azithromycin resembles erythromycin in its antimicrobial spectrum, but an extraordinarily prolonged elimination half-time (68 hours) permits once-a-day dosing for 5 days.
X. Clindamycin resembles erythromycin in antimicrobial activity, but it is more active against many anaerobes. Because severe pseudomembranous colitis can be a complication of clindamycin therapy, this drug should be used only to treat infections that cannot be adequately treated by less toxic antimicrobials.
A. Side Effects
1. Clindamycin produces prejunctional and postjunctional effects at the neuromuscular junction, and these effects cannot be readily antagonized with calcium or anticholinesterase drugs.
2. Large doses of clindamycin can induce profound and long-lasting neuromuscular blockade in the absence of nondepolarizing muscle relaxants and after full recovery from the effects of succinylcholine has occurred.
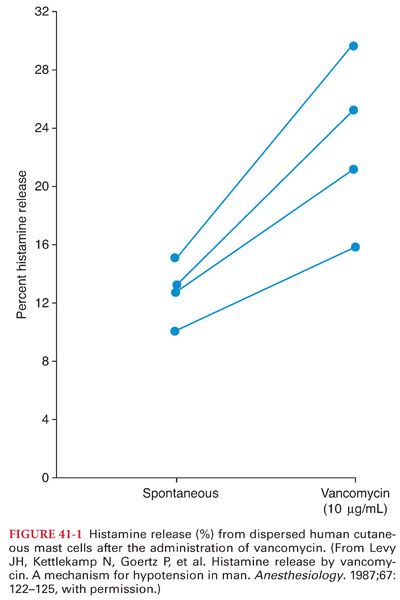
XI. Vancomycin is a bactericidal glycopeptide antimicrobial that impairs cell wall synthesis of gram-positive bacteria. The oral route of administration is used only for the treatment of staphylococcal enterocolitis and antimicrobial-associated pseudomembranous enterocolitis (poorly absorbed from the gastrointestinal tract). Vancomycin is administered IV for the treatment of severe staphylococcal infections or streptococcal or enterococcal endocarditis in patients who are allergic to penicillins or cephalosporins. Concomitant administration of an aminoglycoside is often necessary when vancomycin is used in the treatment of enterococcal endocarditis. Vancomycin is the drug of choice in the treatment of infections caused by methicillin-resistant S. aureus (prosthetic heart valve endocarditis). When vancomycin is administered IV, the recommendation is to infuse the calculated dose (10 to 15 mg/kg) over 60 minutes to minimize the occurrence of drug-induced histamine release and hypotension (begin 2 hours prior to surgery for prophylaxis) (Fig. 41-1). Infusion over 60 minutes produces sustained plasma concentrations for up to 12 hours. Vancomycin is principally excreted by the kidneys, with 90% of a dose being recovered unchanged in urine.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


