Drugs for Systemic Fungal Infections
Amphotericin B
Amphotericin B continues to be an important drug for the treatment of systemic fungal infections. However, several azoles and echinocandins are proving to be just as effective in some systemic mycoses with less risk of toxic effects.
Classification and Pharmacokinetics
Amphotericin B is a polyene antibiotic related to nystatin. Amphotericin is poorly absorbed from the gastrointestinal tract and is usually administered intravenously as a nonlipid colloidal suspension, as a lipid complex, or in a liposomal formulation. The drug is widely distributed to all tissues except the central nervous system (CNS). Elimination is mainly via slow hepatic metabolism; the half-life is approximately 2 wk. A small fraction of the drug is excreted in the urine; dosage modification is necessary only in extreme renal dysfunction. Amphotericin B is not dialyzable.
Mechanism of Action
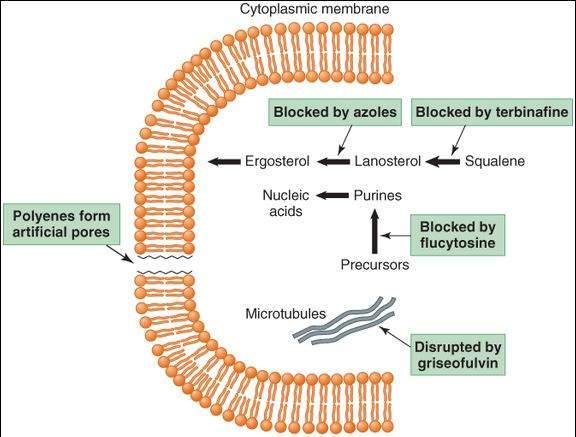
The fungicidal action of amphotericin B is due to its effects on the permeability and transport properties of fungal membranes. Polyenes are molecules with both hydrophilic and lipophilic characteristics (ie, they are amphipathic). They bind to ergosterol, a sterol specific to fungal cell membranes, and cause the formation of artificial pores (Figure 48-1). Resistance, though uncommon, can occur via a decreased level of or a structural change in membrane ergosterol.
FIGURE 48-1
Targets of antifungal drugs. Except for flucytosine (and possibly griseofulvin, not shown), all available antifungal drugs target the fungal cell membrane or cell wall.
(Reproduced, with permission, from Katzung BG, editor: Basic & Clinical Pharmacology, 11th ed. McGraw-Hill, 2009: Fig. 48-1.)
Clinical Uses
Amphotericin B is one of the most important drugs available for the treatment of systemic mycoses and is often used for initial induction regimens before follow-up treatment with an azole. It has the widest antifungal spectrum of any agent and remains the drug of choice, or codrug of choice, for most systemic infections caused by Aspergillus, Blastomyces,Candida albicans, Cryptococcus, Histoplasma, and Mucor. Amphotericin B is usually given by slow intravenous infusion, but in fungal meningitis intrathecal administration, though dangerous, has been used. Local administration of the drug, with minimal toxicity, has been used in treatment of mycotic corneal ulcers and keratitis.
Toxicity
Infusion Related
Adverse effects related to intravenous infusion commonly include fever, chills, muscle spasms, vomiting, and a shock-like fall in blood pressure. These effects may be attenuated by a slow infusion rate and by premedication with antihistamines, antipyretics, meperidine, or glucocorticoids.
Dose Limiting
Amphotericin B decreases the glomerular filtration rate and causes renal tubular acidosis with magnesium and potassium wasting. Anemia may result from decreases in the renal formation of erythropoietin. Although concomitant saline infusion may reduce renal damage, the nephrotoxic effects of the drug are dose-limiting. Dose reduction (with lowered toxicity) is possible in some infections when amphotericin B is used with flucytosine. Liposomal formulations of amphotericin B have reduced nephrotoxic effects, possibly because of decreased binding of the drug to renal cells.
Neurotoxicity
Intrathecal administration of amphotericin B may cause seizures and neurologic damage.
Flucytosine (5-Fluorocytosine [5-FC])
Classification and Pharmacokinetics
5-FC is a pyrimidine antimetabolite related to the anticancer drug 5-fluorouracil (5-FU). It is effective orally and is distributed to most body tissues, including the CNS. The drug is eliminated intact in the urine, and the dose must be reduced in patients with renal impairment.
Mechanism of Action
Flucytosine is accumulated in fungal cells by the action of a membrane permease and converted by cytosine deaminase to 5-FU, an inhibitor of thymidylate synthase (Figure 48-1). Selective toxicity occurs because mammalian cells have low levels of permease and deaminase. Resistance can occur rapidly if flucytosine is used alone and involves decreased activity of the fungal permeases or deaminases. When 5-FC is given with amphotericin B, or triazoles such as itraconazole, emergence of resistance is decreased and synergistic antifungal effects may occur.
Clinical Uses
The antifungal spectrum of 5-FC is narrow; its clinical use is limited to the treatment, in combination with amphotericin B or a triazole, of infections resulting from Cryptococcus neoformans, possibly systemic candidal infections and chromoblastomycosis caused by molds.
Toxicity
Prolonged high plasma levels of flucytosine cause reversible bone marrow depression, alopecia, and liver dysfunction.
Azole Antifungal Agents
Classification and Pharmacokinetics
The azoles used for systemic mycoses include ketoconazole, an imidazole, and the triazoles fluconazole, itraconazole, and voriconazole. Oral bioavailability is variable (normal gastric acidity is required). Fluconazole and voriconazole are more reliably absorbed via the oral route than the other azoles. The triazoles are available in both oral and intravenous formulations. The drugs are distributed to most body tissues, but with the exception of fluconazole, drug levels achieved in the CNS are very low. Liver metabolism is responsible for the elimination of ketoconazole, itraconazole, and voriconazole. Inducers of drug-metabolizing enzymes (eg, rifampin) decrease the bioavailability of itraconazole. Fluconazole is eliminated by the kidneys, largely in unchanged form.
Mechanism of Action
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree