http://evolve.elsevier.com/Edmunds/NP/
Anticonvulsant, or antiepileptic drugs (AEDs) are the medications used to control seizures. Traditionally, these drugs were divided into five classes, including hydantoins, gamma-aminobutyric acid (GABA) analogs, succinimides, barbiturates, and benzodiazepines, plus a miscellaneous class. In addition, the diuretic acetazolamide is used adjunctively to manage seizures. These medications may be used alone or in combination, but the different medication classes are unrelated chemically. Currently, the medications tend to be classified as old drugs, which include the first five classes of drugs plus carbamazepine from the miscellaneous class. The remainder of the miscellaneous class consists of the newer drugs.
Over time, these drug classifications have become less popular, with many clinicians preferring to classify medications as older or newer drugs. The newer drugs are not particularly more effective, but they may have fewer side effects or drug interactions because they are less involved in the induction or inhibition of hepatic P450 enzyme systems. Some of the newer drugs are also used for treating patients with problems other than seizures, such as peripheral neuropathy and pain management. Many of the newer AEDs are approved for adjunctive use only; not all of these are included in this chapter.
In general, seizure disorders are managed in specialists’ offices rather than in primary care settings. The primary care provider often monitors these patients once they are stable and may see them for other acute or chronic medical conditions.
Therapeutic Overview
Pathophysiology and the Disease Process
A seizure is an alteration in behavior, function, and/or consciousness that results from an abnormal electrical discharge of neurons in the brain. Epilepsy, or the term seizure disorder, is used to describe chronic unprovoked recurrent seizures. Seizures are classified according to clinical presentation and EEG characteristics. The International Classification of Seizures by the Commission on Classification and Terminology of the International League Against Epilepsy is summarized in Table 45-2. The treatment chosen for seizure disorder depends on the type of seizure; thus, the correct diagnosis of seizure disorder is imperative. Drugs appear in the table in the order in which they usually are initiated for each type of seizure.
TABLE 45-2
Seizure Classification and Recommended Medication Therapy
| Seizure Type | Description | Drug∗ |
| Simple partial | Focal motor or sensory symptoms; reflects area of brain affected; no change in consciousness | phenytoin carbamazepine valproic acid phenobarbital topiramate gabapentin oxcarbazepine Adjunct—lamotrigine Adjunct—pregabalin Adjunct—tiagabine Adjunct—zonisamide |
| Complex partial | Characterized by an aura, followed by impaired consciousness with automatisms, usually originating from temporal lobe | carbamazepine phenytoin phenobarbital valproic acid gabapentin |
| Secondarily generalized | Simple or complex partial seizures that progress to generalized tonic-clonic seizures | phenytoin carbamazepine phenobarbital valproic acid |
| Generalized tonic-clonic | Formerly “grand mal”; sudden loss of consciousness with tonic-clonic motor activity, postictal state of confusion, drowsiness, and headache | phenytoin carbamazepine phenobarbital valproic acid topiramate |
| Absence | Formerly “petit mal”; brief (<30 seconds) episodes of unresponsiveness characterized by staring, blinking, or facial twitching | ethosuximide valproic acid clonazepam |
| Myoclonic | Series of brief jerky contractions of specific muscle groups | oxcarbazepine zonisamide clonazepam |
| Refractory | Unable to control with other medications | felbamate levetiracetam |
∗Listed in order currently recommended for use, although these recommendations frequently change.
A seizure is a symptom of an underlying brain disorder—not a disease itself. However, the origin of most seizure disorders is unknown. Underlying pathologic conditions, such as tumor, subarachnoid hemorrhage, or hematoma, must be ruled out. Not all behaviors that appear to be seizure activity are seizures. The differential diagnosis for the patient who presents with a history of “seizure” is lengthy. The practitioner must consider systemic, metabolic, and neurologic factors; trauma; brain infection; and behavioral conditions that may cause altered consciousness or behavior. These include syncope, hypoglycemia, cardiac arrhythmia, transient ischemic attack, narcolepsy, psychogenic seizures, alcohol withdrawal, and attention-deficit disorder. New-onset idiopathic epilepsy is unusual in the elderly, who are more likely to have an underlying pathologic condition or metabolic disorder. Of the neurologic disorders, seizures are most common in children. The incidence is highest in children younger than 3. In approximately one in five children who have a seizure, epilepsy will develop.
Seizure most frequently presents in a patient with a known seizure disorder who fails to take adequate suppressive medication. Adherence issues are common with antiepileptic medications; however, concurrent illnesses, changes in medication, or addition of other medications frequently may have an impact on the metabolism of AEDs, some of which have a very narrow therapeutic window.
Assessment
Make sure the episode is actually a seizure. Question patients about what they remember before, during, and after the event. It is usually necessary to question witnesses of the seizure to gain additional information, particularly about what happens during the seizure, the length of the seizure, postictal state, and so forth. Next, try to determine the cause of the seizure. Rule out metabolic disorders, trauma, tumors or other space-occupying lesions, vascular disease, degenerative disorders, migraine with aura, tic disorders, and infectious disease. It is very clear that the origin of seizures varies by age group; the clinician should be alert to this variation when treating patients. For the initial seizure, assessment and testing may be extensive. For a patient with a known seizure disorder, the focus is on what triggered that particular seizure.
Mechanism of Action
For most of these drugs, the exact mechanism for reducing seizure activity is not clearly understood. All increase the threshold of the CNS to convulsive stimuli or inhibit the spread of seizure activity. What is known about the specific mechanisms of action for each class follows.
Hydantoins
The primary site of action appears to be the primary motor cortex, where spread of seizure activity is inhibited. Phenytoin prolongs the effective refractory period by blocking neuronal sodium channels. It stabilizes the threshold against hyperexcitability caused by excessive stimulation or environmental changes and reduces the maximal activity of brainstem centers responsible for the tonic phase of grand mal seizures. It also exhibits antiarrhythmic properties, similar to those of quinidine or procainamide. Although it has little effect on the electrical excitability of cardiac muscle, phenytoin decreases the force of contraction, depresses pacemaker action, and improves atrioventricular conduction, particularly when it has been depressed by digitalis glycosides.
GABA Analogs
The mechanism of action of valproic acid and the newer GABA analogs is not clearly understood. All of these drugs are chemically unrelated, but they increase the actions of GABA, an inhibitory neurotransmitter. They may inhibit the voltage-dependent sodium channel, thereby stabilizing the neuronal membranes. Some of the newer GABA analogs have several actions, including augmentation of GABA, blockage of voltage NA channels, antagonism of glutamate, and modulation of calcium channels.
Carbamazepine
Carbamazepine is chemically similar to the tricyclic antidepressants. It is unrelated structurally to the other analeptics. Its action is similar to that of phenytoin; it limits seizure propagation by blocking postsynaptic transmission.
Barbiturates
Phenobarbital works by inhibiting depolarization of neurons by binding to the GABA receptor, which enhances the transmission of chloride ions. Barbiturates also increase the threshold for electrical stimulation of the motor cortex. Primidone is metabolized to phenobarbital and phenylethylmalonamide (PEMA), which have antiepileptic activity. PEMA may potentiate the activity of phenobarbital.
Benzodiazepines
Benzodiazepines work similarly to barbiturates, but they also increase the number of chloride channels while facilitating transmission of chloride ions. This suppresses the spread of seizure activity but does not abolish abnormal discharge from a focus. Initiate maintenance antiepileptic therapy after seizures have stopped.
Carbamazepine
Carbamazepine is chemically similar to the tricyclic antidepressants. It is unrelated structurally to the other antiepileptics. Its action is similar to that of phenytoin; it limits seizure propagation by blocking postsynaptic transmission.
Gabapentin
Gabapentin acts on voltage-gated calcium channels specifically possessing the alpha-2-delta-1 subunit. This channel appears to be located presynaptically and may modulate the release of excitatory neurotransmitters that participate in the genesis of epilepsy and nociception. Newer extended-release tablets Gralise or Horizant are available. These drugs are not interchangeable with each other or gabapentin because of differing pharmacokinetic profiles.
Pregabalin
Pregabalin binds to the alpha-2-delta subunit of voltage-gated calcium channels within the CNS, inhibiting excitatory neurotransmitter release.
Treatment Principles
Evidence-Based Recommendations
Cardinal Points of Treatment
Pharmacologic Treatment
The decision to initiate antiepileptic therapy must be made with consideration of several variables; most important are the consequences of seizure recurrence for the patient. These consequences are mainly psychosocial and may depend on age, employment, family responsibility, and access to transportation. Make the decision about drug therapy in consultation with the primary care practitioner, the neurologist, the patient, and the family.
A seizure is usually self-limiting in its physical consequences unless the patient suffers physical injury during the seizure. However, research has shown that people who have seizures are at increased risk of sudden death; slowing of cognition and loss of short-term memory after a seizure; and the heavy emotional burden carried by patients who fear they suddenly may have a seizure. Patients who have seizures must live with state regulations such as those that limit their driving, require careful family planning, and control their environment for maximum safety during a seizure.
The goal of medical treatment is to control seizures and allow patients to return to their usual activities. Ideally, this should be done with a single medication that has no significant side effects. This is achieved in about one half of patients.
The medication to use depends on the type of seizure (see Table 45-1). A neurologist generally makes this decision. The medication is started at a low dose and gradually is increased until seizures are controlled or the patient exhibits adverse effects. If the patient continues to experience seizures despite the highest-tolerated dose, a second drug is added gradually. Then the first drug is withdrawn gradually.
The use of complementary or alternative medicine may interact with other antiepileptic drugs and must be used cautiously and with the knowledge of the health care provider. See Table 45-3 for a list of some of these potential interactions.
TABLE 45-3
 Complementary and Alternative Therapies
Complementary and Alternative Therapies
Herbal Interactions with Common Antiepileptics
| Product | Comments |
| Bitter melon | Potential interactions with insulin, oral hypoglycemics |
| Chamomile (German or Roman) | May increase effect of antiepileptics such as phenytoin, valproic acid, and barbiturates |
| Ginkgo | Potential interaction with anticoagulants, aspirin, NSAIDs, antiplatelet drugs; may interact with MAO inhibitors and other antidepressants, acetylcholinesterase inhibitors, antihypertensives, insulin, trazodone. High doses decrease effectiveness of antiepileptic medications. |
| Gymnema | Potential interactions with insulin, oral hypoglycemics |
| Horsetail | Diuretic effect may enhance action of phenytoin |
| Kava kava | May increase the effects of medications used to treat seizures |
| Milk thistle | May interfere with phenytoin |
| Passionflower | May increase sedative effects of phenytoin, barbiturates |
| Siberian ginseng | Increases sedative effects of barbiturates |
| Skullcap (American and Chinese) | Increases sedative effect of barbiturates, benzodiazepines, drugs used to treat insomnia, alcohol, phenytoin, valproic acid |
| St. John’s wort | May increase the sedation effect of phenytoin and valproic acid, barbiturates |
Discontinuation of antiepileptic medication may be considered in patients who have been seizure free for longer than 2 years. An EEG should be obtained before medication is withdrawn. The recurrence rate is usually about 40%, with most seizures occurring in the first year after medication is discontinued. The same psychosocial factors and risks of seizure recurrence for the individual patient should be considered when antiepileptics are withdrawn as when therapy is initiated.
No evidence suggests that prophylactic antiepileptic treatment prevents epilepsy. It is common practice to give an antiepileptic, usually phenytoin, at the time of neurosurgery and after head trauma. Most survivors of brain surgery or head trauma do not later develop epilepsy. Phenytoin given at therapeutic doses during the week after severe head trauma does suppress seizures. This treatment is appropriate when the brain is swollen and cerebral blood flow may be compromised. A seizure could further compromise cerebral blood, leading to increased swelling and intracranial pressure. The same treatment can be applied to the perioperative period. However, no evidence indicates that antiepileptics prevent later epilepsy; therefore, it is not necessary to give long-term antiepileptics to these patients.
Those individuals who have status epilepticus (SE) may experience true medical emergencies that the primary practitioner must be prepared to recognize and manage. For practical purposes, SE is defined as two or more seizures that occur without complete recovery of neurologic function between seizures or as continuous seizure activity for longer than 5 minutes. Therapy should be initiated for any generalized tonic-clonic convulsive seizure activity (formerly called grand mal). Most generalized convulsive seizures last less than 2 minutes. Thus, the practitioner who witnesses a generalized convulsive seizure for longer than 2 minutes should be prepared to initiate emergency procedures. These include activating emergency medical services, such as by calling 911, and protecting the airway. Emergency personnel should initiate intravenous access and administer intravenous benzodiazepines (lorazepam or diazepam) as protocols permit.
Phenobarbital is effective for the prevention of febrile seizures in children, although long-term prophylactic treatment for febrile seizures is not recommended by the American Academy of Pediatrics, which has concluded that the risks of long-term treatment outweigh the benefits. In general, treatment is reserved for those children at greatest risk for future neurologic problems because of seizures; these include children with febrile seizures before 18 months of age, those with neurologic dysfunction or severe developmental delays, those with complex seizures, and those who have seizures with a focal component that last longer than 15 minutes.
Although older-generation agents (AEDs) such as carbamazepine, phenytoin, and valproic acid continue to be used widely in the treatment of epilepsy, these drugs have important shortcomings, such as highly variable and nonlinear pharmacokinetics, a narrow therapeutic index, suboptimal response rates, and a propensity to cause significant adverse effects and drug interactions. Compared with older agents, some of the new AEDs offer appreciable advantages in terms of less variable kinetics and, particularly in the cases of gabapentin, levetiracetam, and vigabatrin, a lower drug interaction potential. Currently, new-generation antiepileptics are used primarily as adjunctive therapy for patients who are refractory to older agents. However, because of advantages in terms of tolerability and ease of use, some of these drugs are used increasingly for first-line management in certain subgroups of patients. As the result of serious toxicity risks, felbamate and vigabatrin should be prescribed only for patients who are refractory to other drugs. In the case of vigabatrin, however, first-line use may be justified in infants with spasms.
Because hepatic metabolism is often the cause of pharmacokinetic drug interactions, enzyme-inducing drugs such as phenytoin, phenobarbital, and carbamazepine readily enhance the metabolism of other antiepileptics. However, many of the newer agents do not undergo hepatic metabolism; this allows for reduced drug–drug interactions. Enzyme-inducing antiepileptics also enhance the metabolism of many other drugs (e.g., oral contraceptives, antidepressants, warfarin) so that the therapeutic efficacy of coadministered drugs is lost unless the dosage is increased.
An increased risk of suicidal thoughts/behavior has been observed for most antiepileptics. Monitor all patients for notable changes in behavior that might indicate suicidal thoughts or depression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


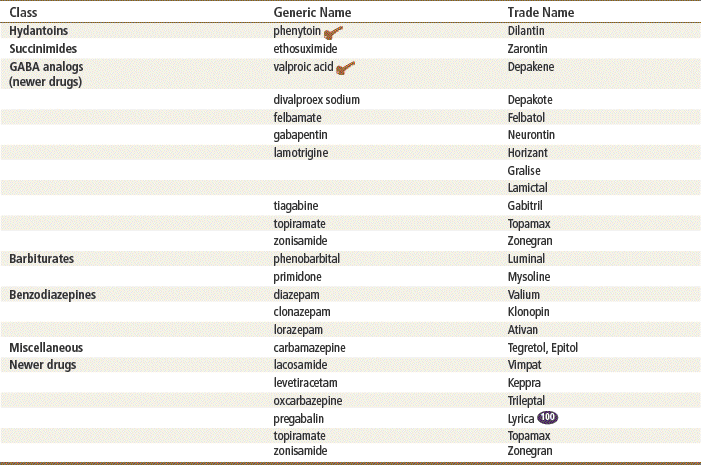
 Top 100 drug;
Top 100 drug;  key drug.
key drug.