

A. Pharmacokinetics (see Table 13-4)
1. All antiepileptic drugs are administered once daily or more frequently.
2. Because of their ability to induce or inhibit drug metabolism, all antiepileptic drugs (except gabapentin, levetiracetam, vigabatrin) may be associated with pharmacokinetic drug interactions in which plasma drug concentrations and resulting pharmacologic effects of concomitantly administered drugs may be altered.
3. Such drug interactions should be anticipated in all patients receiving antiepileptic drugs and subsequently receiving drugs for other purposes.
B. Drug Interactions Related to Protein Binding
1. Salicylates that compete for protein-binding sites of highly bound antiepileptic drugs (phenytoin, valproate, carbamazepine) can displace the bound drug and lead to increases in the plasma concentration of pharmacologically active antiepileptic drug.
2. Hypoalbuminemia, as may accompany renal or hepatic disease or malnutrition, can result in increased plasma concentrations of unbound antiepileptic drug, resulting in toxicity despite therapeutic plasma concentrations.
C. Drug Interactions Related to Accelerated Metabolism
1. Enzyme-inducing antiepileptic drugs that accelerate metabolism (carbamazepine, lamotrigine, oxcarbazepine, phenobarbital, phenytoin, topiramate, and primidone) may accelerate the metabolism of estrogen and progesterone and thus render oral contraceptives ineffective at usual doses.
2. Patients being treated with antiepileptic drugs have increased dose requirements for thiopental, propofol, midazolam, opioids, and nondepolarizing neuromuscular blocking drugs.
D. Principles of Dosing
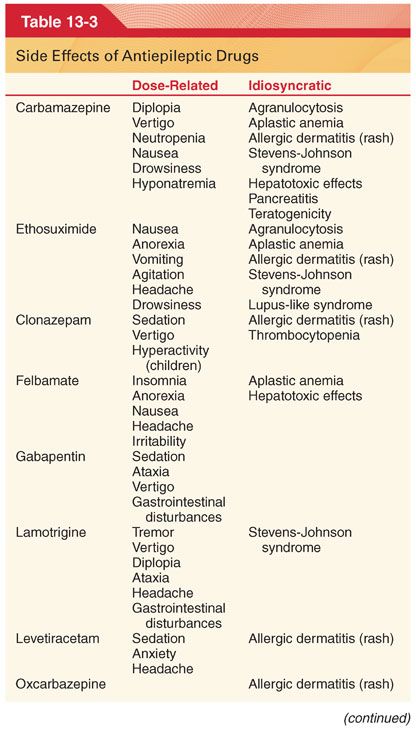
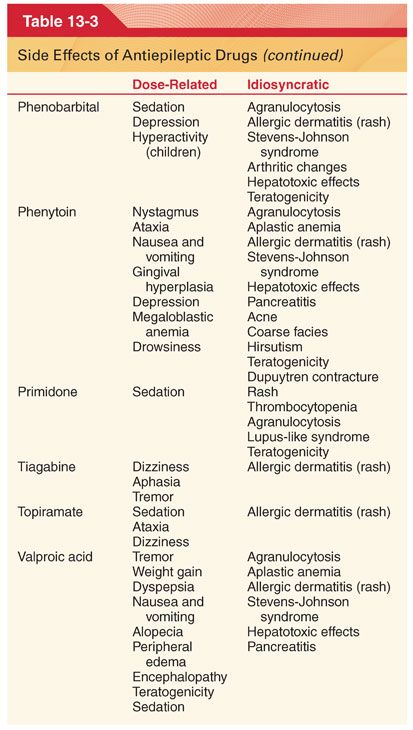
1. The initial dose is that which is high enough to expect clinical effect but low enough to avoid significant side effects (see Table 13-3).
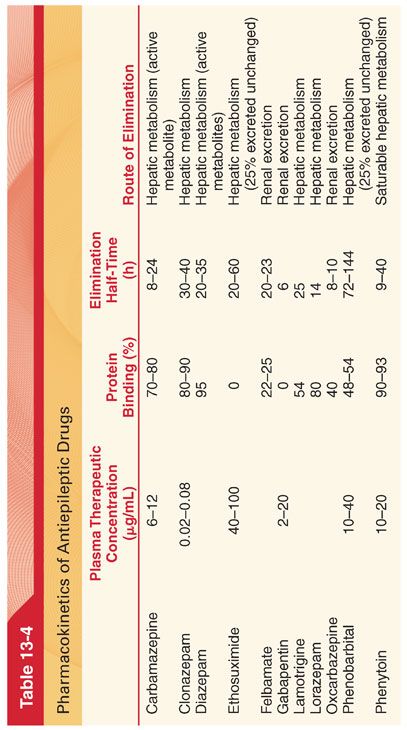
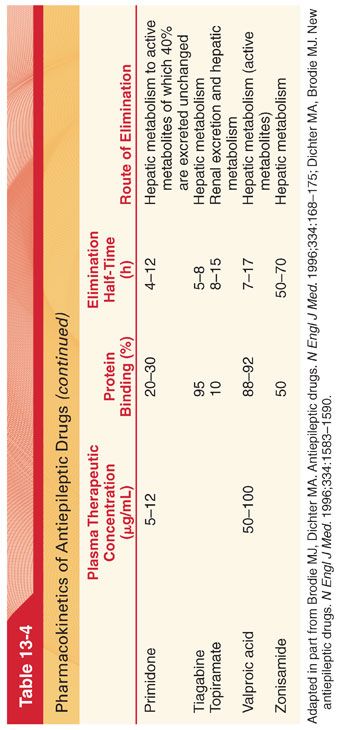
2. To maintain plasma drug concentrations in a therapeutic range, equal doses of the antiepileptic drug are often administered at intervals equivalent to less than one elimination half-time of the drug (see Table 13-4).
E. Plasma Concentrations and Laboratory Testing. Phenytoin is the only agent for which monitoring is routinely recommended due to its nonlinear saturation dose kinetics.
II. Mechanism of Seizure Activity. The reason for the high frequency and synchronous firing in a seizure focus is usually unknown (possible explanations include local biochemical changes, ischemia, loss of cellular inhibitory systems, infections, and head trauma). Once initiated, a seizure is most likely maintained by reentry of excitatory impulses in a closed feedback pathway that may not even include the original seizure focus.
III. Mechanism of Drug Action. The mechanism of action of antiepileptic drugs is incompletely understood. It is commonly presumed that antiepileptic drugs control seizures by decreasing neuronal excitability or enhancing inhibition of neurotransmission.
IV. Major Antiepileptic Drugs (see Table 13-2)
A. Adverse Side Effects. Antiepileptic drugs may potentially produce numerous and varied adverse side effects (see Table 13-3).
B. Maternal Epilepsy
1. Seizures during pregnancy can result in significant morbidity and mortality to both mother and fetus, making seizure control during this period imperative (fetal organogenesis is largely complete by 8 weeks).
2. Significant teratogenicity may occur during this period if pregnancy is not detected early enough to permit discontinuation of potentially teratogenic medications.
C. Carbamazepine is effective for suppression of nonconvulsive and convulsive partial seizures and is useful in the management of patients with trigeminal neuralgia and glossopharyngeal neuralgia.
1. Pharmacokinetics (see Table 13-4)
2. Side Effects (see Table 13-3). Sedation, vertigo, diplopia, nausea, and vomiting are the most frequent side effects of this drug. Aplastic anemia, thrombocytopenia, hepatocellular and cholestatic jaundice, oliguria, hypertension, and cardiac dysrhythmias are rare but potentially life-threatening complications.
a. In addition to inducing its own metabolism, carbamazepine can accelerate the hepatic oxidation and conjugation of other lipid-soluble drugs.
b. The most common interaction is with oral contraceptive pills, and most women require an increase in the daily dose of estrogen.
D. Ethosuximide is the drug of choice for suppression of absence (petit mal) epilepsy in patients who do not also have tonic-clonic seizures.
1. Pharmacokinetics (see Table 13-4)
2. Side Effects (see Table 13-3). Toxicity of ethosuximide is low, manifesting most often as gastrointestinal intolerance (nausea, vomiting) and central nervous system (CNS) effects (lethargy, dizziness, ataxia, photophobia).
E. Felbamate is not used as a first-line drug for treatment of seizures (toxicity) but rather is reserved for patients with intractable epilepsy.
1. Pharmacokinetics (see Table 13-4)
2. Side Effects (see Table 13-3). Serious side effects include aplastic anemia and hepatotoxicity. Monitoring of treated patients with complete blood counts and liver function tests is indicated.
F. Gabapentin is an analog of γ-aminobutyric acid (GABA) that increases synaptic GABA concentrations. Gabapentin induces dose-related sedation and it has efficacy in the treatment of anxiety, panic, and major depression. Despite its multiple other uses, gabapentin has limited efficacy in the treatment of epilepsy.
G. Lamotrigine has a broad spectrum of activity and is effective when used alone or in combination in adults who have partial seizures or generalized seizures and in children with Lennox-Gastaut syndrome (see Table 13-3).
1. Drugs that induce hepatic microsomal enzymes (phenobarbital, phenytoin, and carbamazepine) decrease the elimination half-time of lamotrigine by about 50%, necessitating a higher dose.
2. Conversely, valproic acid slows the metabolism of lamotrigine and extends its elimination half-time to about 60 hours.
H. Phenytoin is the prototype of the hydantoins and is effective for the treatment of partial and generalized seizures. Available in oral and intravenous (IV) preparations, phenytoin may be administered acutely to achieve effective plasma concentrations within 20 minutes. This drug has a high therapeutic index, and its administration is not accompanied by excessive sedation.
1. Mechanism of Action. Phenytoin regulates neuronal excitability and thus the spread of seizure activity from a seizure focus by regulating sodium and possibly calcium ion transport across neuronal membranes (stabilizing effect on cell membranes is relatively selective for the cerebral cortex).
2. Pharmacokinetics (see Table 13-4)
a. The initial daily adult oral dosage (intramuscular administration is not recommended) is 3 to 4 mg/kg (doses of >500 mg daily are rarely tolerated).
b. The rate of IV administration of phenytoin should not exceed 50 mg per minute in adults and 1 to 3 mg/kg/min (or 50 mg per minute, whichever is slower) in pediatric patients because of the risk of severe hypotension and cardiac arrhythmias.
3. Plasma Concentrations
a. Control of seizures is usually obtained when plasma concentrations of phenytoin are 10 to 20 μg/mL.
b. In the control of digitalis-induced cardiac dysrhythmias, phenytoin, 0.5 to 1.0 mg/kg IV, is administered every 15 to 30 minutes until a satisfactory response is achieved or a maximum dose of 15 mg/kg is administered.
4. Protein Binding. Phenytoin is bound approximately 90% to plasma albumin. A greater fraction of phenytoin remains unbound in neonates, in patients with hypoalbuminemia, and in uremic patients.
5. Metabolism of phenytoin to inactive metabolites is by hepatic microsomal enzymes that are susceptible to stimulation or inhibition by other drugs.
6. Side Effects (Table 13-5)

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


