http://evolve.elsevier.com/Edmunds/NP/
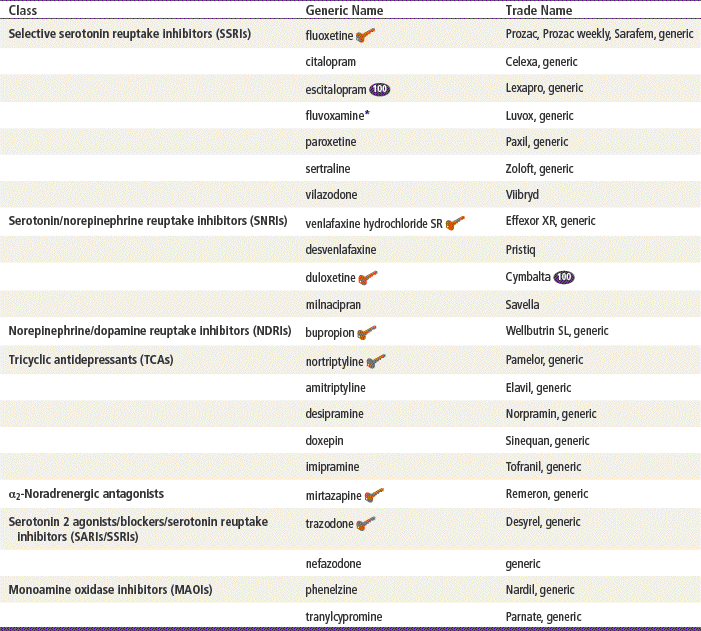
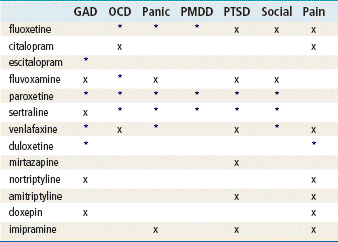
 Top 100 drug;
Top 100 drug;  key drug. Drugs listed in order of common use.
key drug. Drugs listed in order of common use.
∗Not FDA approved as an antidepressant.
A CDC report found an almost 400% increase in the rate of antidepressant use in the United States since 1988, making antidepressants the third most common prescription medication taken by people of all age groups from 2005 to 2008. An estimated 11% of Americans aged 12 or older use the medications, and those between 18 and 44 years old were the most frequent antidepressant users.
Many people are depressed because they are facing hardship and problems but without having a clinically significant depression that requires treatment. Many antidepressants have been found to be prescribed for routine complaints for patients with no mental health diagnosis. Direct to consumer advertising has driven many patients to insist on antidepressant therapy, although they may not fully understand the risks.
Researchers suggest many patients who have depression are reluctant to discuss this with clinicians. Some early studies suggest patients who have had depression in middle or later life may have a 34% higher absolute risk of having a stroke, and diabetes combined with depression seems to increase the risk of dementia. Therefore, patients should be correctly diagnosed. Recent studies that reevaluated the results of 234 previously published studies on newer antidepressant effectiveness found no significant difference in the efficacy of new antidepressants, while their effect still varies for every patient. Findings showed that the drugs differed in terms of how fast they took effect and their side effects. The conclusion was that contrary to claims of the drug industry, scientific evidence does not support the choice of one drug over another based solely on better effectiveness. Dual-acting antidepressants (SNRIs) are no more effective than mono-acting antidepressants (SSRIs).
SSRIs are the most commonly used first-line therapy. They produce only a marginal response when compared with placebo, or at best a 20% better response rate than placebo in some trials. They are similar in action and response, and the one used varies depending on the clinician’s familiarity with the medication and individual patient response. Most SSRIs have a somewhat questionable safety profile, particularly when weight gain and risk of suicide are considered.
The SNRIs bupropion and mirtazapine are also commonly used in primary care. All depressants are heavily prescribed by primary care providers, including so-called “adjuncts,” although there are marginal objective data supporting their use.
MAOIs are used as third-line agents in cases of refractory and atypical depression. Many TCAs are available, but this chapter mentions only those in common use. These agents produce acceptable results in patients who have not responded to one of the other antidepressants. They also are used when cost is a concern and as an adjunct treatment for depression and pain. Nefazodone is used only when other agents have failed; it carries a “black box” warning for liver toxicity. Trazodone is used largely as an adjunct or to induce sleep. MAOIs are used as third-line agents in cases of refractory and atypical depression. This chapter mentions MAOIs briefly but does not discuss them in detail because they generally are prescribed by a psychiatrist—not a primary care provider.
Therapeutic Overview
The critical difference between neurons is the neurotransmitter that their receptors recognize teleologically. Three important types of monoaminergic neurons in the brain modulate mood. The first are the serotonergic neurons, also called 5-HT, which use serotonin as their neurotransmitter. The second are the noradrenergic (norepinergic) neurons, which use norepinephrine as their neurotransmitter. (This terminology is nonscholarly, but the term has remained in use.) The third group consists of the dopaminergic neurons, which use dopamine as their neurotransmitter. Neurotransmitters can attach to a receptor on the same cell that they were released from (a presynaptic receptor) and affect the activity of that cell. These presynaptic receptors act as autoreceptors. They recognize when a sufficient amount of neurotransmitter is present in the synapse and inhibit further release of the neurotransmitter, operating as a brake. This works as a negative feedback system that regulates the amount of neurotransmitter in the synapse. If the presynaptic receptor is blocked, the receptor will keep the brake from functioning, allowing enhanced release of the neurotransmitter. The receptors can be blocked by an agent that occupies the receptor site but does not act on the site. This prevents the normal neurotransmitter from doing what it usually does.
Serotonergic neurons are located mainly in the area of the brainstem called the raphe nucleus. They regulate anxiety, movements, obsessions and compulsions, appetite and eating behavior, sleep, sexual response, and GI motility. The two major presynaptic serotonergic neuron receptors are 5-HT1A and 5-HT1D. Six major serotonergic postsynaptic receptors have been identified: 5-HT1A, 5-HT1D (the same as presynaptic), 5-HT2A, 5-HT2C, 5-HT3, and 5-HT4. Many more postsynaptic receptors are known, and new ones are being discovered. Stimulation of HT1A (desirable) receptors improves mood and decreases eating disorders. HT2A (undesirable) receptors are associated with anxiety, agitation, panic, decreased libido, sexual dysfunction, myoclonus, impaired sleep, and apathy. Stimulation of 5-HT2C receptors causes anxiety, agitation, and panic. 5-HT3 and 5-HT4 receptors are located in the gut, where they control tone and motility, and their stimulation may cause nausea, vomiting, increased bowel motility, cramps, and diarrhea. Serotonergic neurons have postsynaptic autoreceptors such as 5-HT1A, unlike the catecholamines norepinephrine and dopamine. Serotonergic neurons also have receptors on the cell bodies that enhance serotonin release. Norepinephrine can function as a brake for serotonin release by acting on the NE-α2 receptors that are found on serotonin neurons, as well as on adrenergic neurons.
Noradrenergic neurons are located primarily in the part of the brainstem called the locus coeruleus. The main function of the noradrenergic neurons is attention. They also have a role in control of memory, information processing, emotions, energy, psychomotor function, movement, blood pressure, heart rate, and bladder emptying.
Dopaminergic neurons are located in the substantia nigra, the medial mesencephalic tegmentum, and the hypothalamus. They control movement, primitive emotions, and visceral functions. Dopaminergic neurons have at least five types of receptors, of which D2 receptors are the most studied. The D2 receptor is important in Parkinson’s disease and in schizophrenia. D1 through D4 receptors are affected by some of the atypical antipsychotic drugs. The newer antipsychotics affect mainly D2 receptors.
Many other neurotransmitters are affected by antidepressant medications, causing numerous side effects (Table 47-2). The muscarinic system has two branches: the nicotinic and the cholinergic. Of the two, the cholinergic is by far more important. Cholinergic neurons use acetylcholine. The terms muscarinic and cholinergic often are used interchangeably. The histaminergic, α1-adrenergic, and α2-adrenergic systems also are involved in drug action.
TABLE 47-2
Adverse Effects of Neurotransmitters
| Neurotransmitter | Adverse Effects |
| Serotonin | Anxiety, agitation, anorexia, GI distress, headache, hypotension, sexual dysfunction |
| Norepinephrine | Tachycardia, tremors, sexual dysfunction; augments sympathomimetics |
| Dopamine | Extrapyramidal symptoms, increased prolactin levels, psychosis, insomnia, anorexia, psychomotor activation |
| Acetylcholine | Memory dysfunction, tachycardia, blurred vision, dry mouth, urinary retention, constipation |
| Histamine | Sedation, hypotension, weight gain, allergy |
| α1-Adrenergic | Orthostatic hypotension, dizziness, cardiac conduction disturbance |
| α2-Adrenergic | Priapism |
Pathophysiology
Our understanding of the chemistry behind depression has grown dramatically, but much of it is theoretical, and it remains a very controversial subject. The neurochemical basis of depression is complex and is not the result of any one specific deficit. The function of the hypothalamic-pituitary axis and the involvement of stress-related hormones are increasingly believed to play a role in the development of depression. In reality, researchers do not know exactly what causes depression, even though the norepinephrine system has been studied intensively. Changes occur in the α- and β-adrenergic receptors—receptors that norepinephrine acts upon within the brain. Upregulation of β-receptors is thought to be due to a relative deficiency of norepinephrine at certain critical synapses in the brain. In older studies, measurements of norepinephrine metabolites, such as 3-methoxy-4-hydroxyphenylglycol (MHPG), have been reported as well. A relative lack of serotonin and decreased density of the postsynaptic 5-HT2 receptors have been noted to produce a relative deficiency of serotonin in the brain. A relative lack of dopamine in presynaptic neurons and dopamine transporters has also been observed. Pharmacologic research demonstrates improvement in depression when drugs are administered that increase relative amounts of these chemicals in at best 60% of cases, leaving 40% of patients with an unacceptable response, thereby highlighting the complex pathophysiology and response to pharmacology.
Disease Process
Significant depressive symptoms are seen in nearly 40% of primary care patients, and major depression occurs in up to 10%. In general antidepressants are ineffective in treating depressive symptoms solely and for the most part ineffective in treating mild to moderate depression. Major depression is a common, vastly undertreated, and potentially fatal disease, while mild to moderate depression is vastly overdiagnosed and extremely overtreated and with suboptimal efficacy. Depression is common in postpartum mothers, both young and old. It is a heterogeneous disorder with a wide variety of presentations.
Many mood disorders must be differentiated. See Box 47-1 for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) diagnostic criteria for major depression. The patient with suspected major depression must have these symptoms on a daily basis for a minimum of 2 weeks. In addition, the severity of symptoms must significantly impair the patient’s quality of life and ability to perform activities of daily living (ADLs). Five types of depression or basic clusters of symptoms are known. Cognitive symptoms include memory loss, slowed thoughts, decreased attention, and inability to concentrate. Vegetative symptoms include increased or decreased sleep, altered appetite or weight, psychomotor movement, and decreased sexual function. Physical symptoms consist of fatigue, muscle tension, pain—especially head and stomach—decreased sexual drive, and weakness. Behavioral symptoms include social withdrawal, loss of interest in usual activities, crying, weeping, decreased frustration tolerance, agitation and irritability, phobias, and poor attention to self-care. Emotional symptoms occur as guilt, feelings of worthlessness, suicidal ideation or behavior, disappointment with self, hopelessness, helplessness, anxiety, and delusions or hallucinations.
Another depressive disorder commonly seen in the primary care patient is atypical or “minor” depression. Symptoms of minor depression occur at the same frequency (i.e., daily, or nearly daily for at least 2 weeks) as those of major depression. However, patients with minor depression will have only two to four depressive symptoms that often include weight gain and hypersomnia. This contrasts with major depression, in which weight loss and insomnia are typically seen.
Dysthymia is a depressive disorder in which symptoms are present for at least 2 consecutive years; these include lack of interest, low self-esteem, and low energy. It is present in approximately 3% to 5% of primary care patients and responds poorly if at all to pharmacotherapy.
Bereavement over the loss of a loved one may be associated with symptoms of depression. Despite the findings that 94% of these patients will meet the definition of major depression, only 17% will be treated with antidepressants. Treating bereavement is controversial, as it may also be both harmful and ineffective. During the normal course of grief, 94% of symptoms have been found to resolve in 13 months and often dramatically sooner.
Catatonic depression, comorbid anxiety disorder, and seasonal affective disorder (SAD) are other types of depressive disorder. Other diagnoses that should be considered include depression with alcohol or substance abuse or dependence, prenatal and postpartum depression, comorbid personality disorder, and the depressive phase of bipolar disorder.
The presence of bipolar disorder in the depressive stage always must be considered by the clinician. Treatment of a bipolar patient with antidepressants may induce rapid cycling to manic phase, so careful monitoring is required, particularly if bipolar disorder is suspected but has not yet been confirmed.
The natural history of untreated depression is that of recurrence. Untreated depression may resolve without treatment, but recurrences are common. Risks for recurrence include increased severity and duration of the depression, psychotic symptoms, incomplete recovery between episodes, and prior episodes of depression. If a patient has had three or more episodes of depression, the chance of a relapse is greater than 90%.
Assessment
A complete history and physical examination are essential for the differential diagnosis of depression and for selection of the best medication for the patient. The discussion below is a brief review. Medical illnesses and medications also can contribute to the development of depression (see Tables 47-3 and 47-4). The diagnosis must rule out other factors such as drug or alcohol abuse, use of other medications, and medical conditions. Medical conditions that increase the risk of depression are cancer, chronic lung disease, heart disease, stroke, diabetes, end-stage renal disease, dementia, hypothyroidism, chronic fatigue syndrome, fibromyalgia, systemic lupus erythematosus, anxiety, and panic disorder. Also ask about past episodes of depression and hypomania and a family history of bipolar disorder. See Box 47-2 for a list of risk factors for depression.
TABLE 47-3
Illnesses Commonly Occurring with Depression
| System | Examples |
| Autoimmune disease | Rheumatologic disorders |
| CNS disease | Stroke |
| Dementia | |
| Endocrine system disease | Diabetes |
| Thyroid disorder | |
| Heart disease | Chronic heart failure |
| Myocardial infarction | |
| Malnutrition | Vitamin deficiency |
| Protein/calorie deficiency | |
| Mood disorders and psychiatric conditions | Bipolar disorders |
| Alcohol/drug dependency | |
| Eating disorders | |
| Obsessive-compulsive disorders | |
| Anxiety disorders | |
| Somatization disorders | |
| Personality disorders | |
| Psychosis | |
| Other medical problems | Oncologic/hematologic disease |
| Chronic fatigue syndrome | |
| Infectious disease |
TABLE 47-4
Drug Classes That Produce the Side Effect of Depression
| Drug Class | Specific Examples |
| Antihypertensives | Calcium channel blockers (diltiazem), methyldopa, nifedipine, thiazide diuretics, verapamil |
| Hormones | estrogen, progestins (Norplant), corticosteroids (prednisone, cortisone, ACTH), dapsone |
| Histamine2-receptor blockers | famotidine (Pepcid), cimetidine, metoclopramide, nizatidine |
| Anticonvulsants | Barbiturates, carbamazepine, clonazepam, phenytoin, valproic acid |
| Antiparkinsonian agents | levodopa |
| Cardiac medications | digitalis glycosides, HMG-CoA reductase inhibitors (statins) |
| Antiinfectives | Fluoroquinolone antibiotics, isoniazid, metronidazole, sulfonamides |
| Sedative-hypnotics | Benzodiazepines |
| Antineoplastics | vinblastine |
| NSAIDs | ibuprofen, indomethacin, naproxen, sulindac |
Formal screening tools for depression such as the Zung Self-Assessment Depression Scale, the Beck Depression Inventory, and the General Health Questionnaire (GHQ) are available to the provider. The choice of one tool overanother is a matter of preference. Two questions may elicit depression: “Over the past 2 weeks, have you felt down, depressed, or hopeless?” and “Over the past 2 weeks, have you felt little interest or pleasure in doing things?” Patients who test positive should undergo a complete diagnostic interview for a specific depressive disorder based on DSM-IV criteria.
It is crucial to assess the risk of suicide in the depressed patient. The provider should pay particular attention to the presence of the following:
Mechanism of Action
Increased monoamine neurotransmitters serotonin, norepinephrine, and dopamine may produce an elevated mood, but that does not always signify the successful treatment of depression. Some researchers firmly believe that antidepressants are ineffective because they treat the symptoms, not the depression. All current antidepressants act on neurotransmitter systems by affecting three distinct processes: neurotransmitter degradation, neurotransmitter reuptake (i.e., reabsorption into the releasing cell), and neurotransmitter binding.
Degradation of all three neurotransmitters is accomplished by the monoamine oxidase (MAO) enzyme. The catechol-O-methyltransferase (COMT) enzyme degrades norepinephrine and dopamine. MAO degrades neurotransmitters after reabsorption into the presynaptic neuron. MAOs occur in two forms—MAOa and MAOb—and are present in tissues throughout the body. Drugs that inhibit the enzyme and thus increase the concentrations of neurotransmitters are known as MAOIs.
The next mechanism of action of antidepressants involves inhibiting the reuptake of neurotransmitters in the synapse. Neurotransmitters are removed from the synapse by reuptake pumps on the presynaptic neuron, which takes them up and stores them for further use. Inhibiting this reuptake enhances the amount of neurotransmitter in the synapse. The third mechanism of action of antidepressants involves neurotransmitter receptor binding sensitization. This is a delayed effect.
At the same time, these antidepressants may bind themselves directly to postsynaptic receptors that produce side effects. Such receptors include histaminergic H1, muscarinic, H1-adrenergic, and dopaminergic D2 receptors. The clinical effects that result when these receptors are stimulated by antidepressants may include dizziness, hypotension, tachycardia, and GI disturbances. Histaminergic H1 antagonism has been associated with sedation and drowsiness. Muscarinic-receptor stimulation is responsible for constipation and dry mouth. Stimulation of the H1-adrenergic receptor may be responsible for dizziness and orthostatic hypotension.
All categories of antidepressant medication have a common major mechanism of action. Slight variations within a drug category may be noted. Table 47-5 shows the antidepressants as classified according to mechanism of action. These medications are discussed in terms of the order of current usage. Older medications tend to have additional side effects. Newer antidepressants demonstrate a trend toward more specifically tailored mechanisms of action. Antidepressant therapies that are not categorized as SSRIs, TCAs, or MAOIs also have been developed; these are referred to in the literature as atypical antidepressants. The drugs include bupropion (Wellbutrin), nefazodone (Serzone), and trazodone (Desyrel).
TABLE 47-5
Antidepressant Classification Based on Neurotransmitter
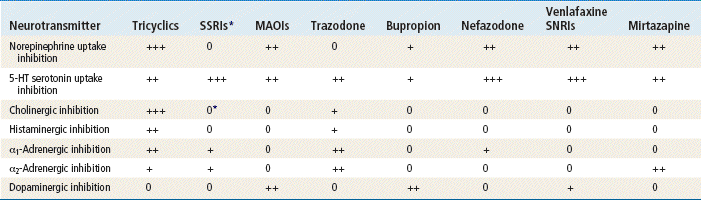
0, No activity; +, weak activity; ++, moderate activity; +++, high activity.
∗Citalopram, fluoxetine, paroxetine, and fluvoxamine have weak cholinergic inhibition.
Selective Serotonin Reuptake Inhibitors
All SSRIs work by increasing the amount of serotonin by blocking the presynaptic serotonin reuptake pump. At least 14 different postsynaptic serotonin receptors are present in humans. Each of these receptors controls a different physiologic and/or psychiatric function. The action of the SSRIs is related to which postsynaptic receptor is stimulated. It is believed that the 5-HT1A receptors are primarily responsible for the antidepressant effect and that the other receptor subtypes may be responsible for some of the side effects of SSRIs.
Serotonin/Norepinephrine Reuptake Inhibitors
Venlafaxine (Effexor) and desvenlafaxine are non–tricyclic antidepressants with dual serotonin and norepinephrine reuptake inhibition and weak reuptake inhibition of dopamine that has demonstrated efficacy in the treatment of depression and produces fewer side effects than are produced by tricyclics. Thus, they act on all three of the monoamine neurotransmitters, as do the TCAs. Different from the TCAs and similar to the SSRIs, venlafaxine has virtually no affinity for muscarinic, histaminergic, or α1-adrenergic receptors. The side effect profile is thus closer to that of the SSRIs than the TCAs. At higher doses, the risk of high blood pressure is increased. Another SNRI, duloxetine hydrochloride (Cymbalta), was approved for the treatment of major depressive disorder. Duloxetine is an inhibitor of serotonin and norepinephrine reuptake and a lesser inhibitor of dopamine reuptake. Milnacipran (a non–FDA-approved antidepressant) is an inhibitor of norepinephrine and serotonin reuptake.
Norepinephrine and Dopamine Reuptake Inhibitors
Bupropion is structurally similar to amphetamine and is considerably different from other antidepressants. Bupropion acts weakly as a norepinephrine and dopamine reuptake inhibitor (NDRI). It uniquely increases dopamine in the nucleus accumbens, which is known as the “reward area” of the brain in smokers. Therefore, bupropion is used for the treatment of smoking cessation.
Tricyclic Antidepressants
The TCAs were discovered in the 1950s and are named for their chemical structure, which contains three rings. They are subdivided further into the tertiary and secondary amines. The secondary amines (desipramine and nortriptyline) are metabolites of the tertiary amines (amitriptyline and imipramine). This results in significant differences in the pharmacokinetic and pharmacodynamic effects of each drug.
TCAs exert their therapeutic effects by blocking (inhibiting) reuptake of norepinephrine and serotonin at the presynaptic neurons. They block the reuptake of dopamine to a lesser degree. Some TCAs block 5-HT receptors—an added therapeutic action. In addition, they block cholinergic, histamine, and α1-adrenergic receptors; this effect is associated with the many side effects of TCAs. TCAs also block sodium channels in the heart and brain, which can cause cardiac arrhythmias and seizures.
Noradrenergic Antagonists
Introduced in 1997, mirtazapine is a tetracyclic compound that is unrelated to the TCAs. It is an α2-antagonist that blocks the α2-receptors on the adrenergic neurons. The α2-receptors are noradrenergic receptors that act as presynaptic autoreceptors. When they detect adequate levels of norepinephrine, they stop norepinephrine release. An α2-antagonist will block the negative feedback loop, raising norepinephrine levels. This same mechanism also will stop norepinephrine from blocking serotonin release, thus raising serotonin levels. The drug blocks three serotonin receptors (i.e., 5-HT2A, 5-HT2C, and 5-HT3) and has antihistaminergic properties.
Serotonin 2 Agonist/Serotonin Reuptake Inhibitors
These agents block 5-HT receptors and serotonin reuptake presynaptically and postsynaptically. Their most powerful action is to block 5-HT receptors. This is called 5-HT antagonism. They block serotonin reuptake, as do the SSRIs, but this is a less potent action. They are weak α1-adrenergic blockers.
Nefazodone is a weak blocker of norepinephrine reuptake inhibitors. It has virtually no affinity for cholinergic, α2-, or β-adrenergic, dopamine D1, D2, or histamine receptors.
Trazodone also blocks histamine1 (H1) receptors, and this causes sedation.
Monoamine Oxidase Inhibitors
The monoamine neurotransmitters dopamine, norepinephrine, and serotonin are broken down by the enzyme MAO. MAOIs are the medications that block this breakdown, causing increased levels of these three neurotransmitters. The first two MAOIs that blocked the MAO enzyme were irreversible, but very new ones are reversible inhibitors of MAO, known as reversible inhibitor monoamines (RIMAs).
Treatment Principles
Evidence-Based Recommendations
The vast majority of clinical trials and meta-analyses have found that all antidepressants are considered equally efficacious. These conclusions are based on several factors, including efficacy, side effects, and overall treatment costs. Thus, the choice of initial antidepressant rests with the provider. In many cases, the SSRIs are chosen as first-line agents because of their side effect profiles and the relative lack of danger associated with overdose. Please note that in clinical trials “efficacy” is measured, while in clinical practice “effectiveness” is actually being observed.
Cardinal Points of Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree