B. The effects of cardiac antiarrhythmic drugs on the action potential and effective refractory period of the cardiac action potential determine the clinical effect of these drugs.
III. Classification
A. Cardiac arrhythmic drugs are most commonly classified into four groups based primarily on the ability of the drug to control arrhythmias by blocking specific ion channels and currents during the cardiac action potential (Tables 21-1 and 21-2).
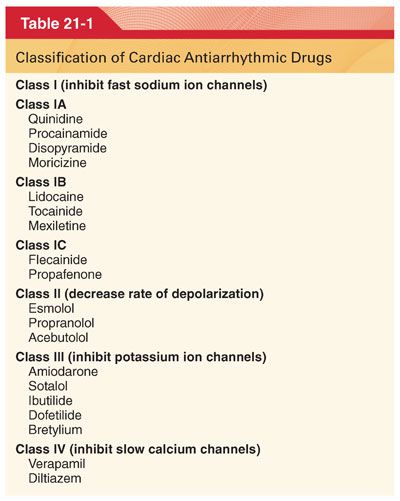

B. Antiarrhythmic drugs also differ in their pharmacokinetics and efficacy in treating specific types of arrhythmias (Tables 21-3 and 21-4).
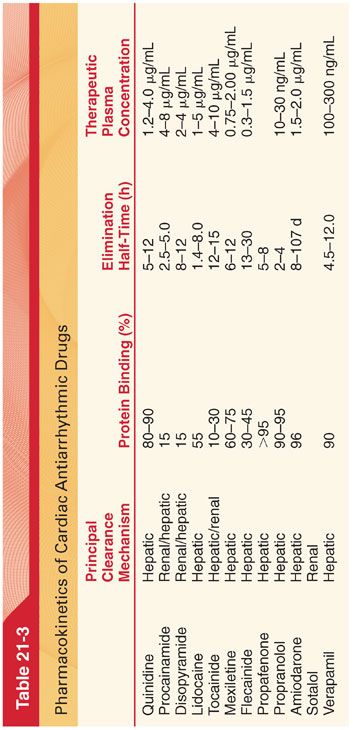
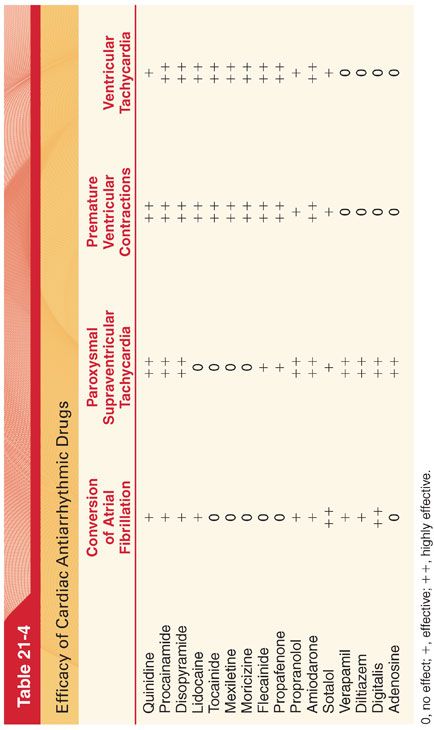
IV. Proarrhythmic effects describe bradyarrhythmias or tachyarrhythmias that represent new cardiac arrhythmias associated with antiarrhythmic drug treatment.
A. Torsades de pointes manifests as prolongation of the QTc interval on the electrocardiogram (ECG).
1. Class IA drugs (quinidine and disopyramide) and class III drugs (amiodarone) prolong the QTc interval by potassium channel blockade providing the setting for torsades de pointes.
2. Drug-induced torsades de pointes is often associated with bradycardia because the QTc interval is longer at slower heart rates.
B. Incessant ventricular tachycardia may be precipitated by drugs that slow conduction of cardiac impulses (class IA and class IC drugs) sufficiently to create a continuous ventricular tachycardia circuit (reentry). Incessant ventricular tachycardia is more likely to occur with high doses of class IC drugs and in patients with a prior history of sustained ventricular tachycardia and poor left ventricular function.
C. Wide complex ventricular rhythm is usually associated with class IC drugs in the setting of structural heart disease.
V. Efficacy and Results of Treatment with Cardiac Antiarrhythmic Drugs
A. Chronic suppression of ventricular ectopy with an antiarrhythmic drug other than amiodarone does not prevent future life-threatening arrhythmias and may increase mortality.
1. Patients treated with class IC drugs experienced a higher incidence of sudden cardiac arrest reflecting the proarrhythmia effects of these drugs.
2. β-Adrenergic antagonists that do not typically suppress ventricular arrhythmias appear to decrease mortality and the risk of life-threatening ventricular arrhythmias.
B. Survivors of cardiac arrest have a high risk of subsequent ventricular fibrillation and treatment of these patients with amiodarone results in fewer life-threatening cardiac events.
C. The proarrhythmic and negative inotropic effects of class IA and class IC drugs precludes their administration to patients with congestive heart failure. In these patients, administration of amiodarone appears to be safe and effective.
VI. Prophylactic Antiarrhythmic Drug Therapy
A. Although commonly used in the past, lidocaine is no longer recommended as prophylactic treatment for patients in the early stages of acute myocardial infarction and without malignant ventricular ectopy.
B. Calcium channel antagonists are not recommended as routine treatment of patients with acute myocardial infarction because mortality is not decreased by these drugs.
C. Data on the ability of magnesium to decrease mortality following myocardial infarction are conflicting. Treatment with magnesium is indicated in patients following an acute myocardial infarction who develop torsades de pointes ventricular tachycardia.
D. In patients with heart failure, amiodarone reduces the risk of sudden cardiac death and therefore represents a viable alternative in patients who are not eligible for or who do not have access to implanted cardiac defibrillator (ICD) therapy for the prevention of sudden cardiac death from arrhythmias.
E. Atrial fibrillation after heart surgery is a common complication that has been associated with prolonged hospitalization and cardiovascular morbidity. Prophylactic therapy with amiodarone, β blockers, sotalol, and magnesium has been effective in reducing the occurrence of atrial fibrillation, length of hospital stay, and cost of hospital treatment and may be effective in reducing the risk of stroke.
VII. Decision to Treat Cardiac Arrhythmias
A. Drug treatment of cardiac arrhythmias is not uniformly effective and frequently causes side effects. The benefit of antiarrhythmic drugs is clearest when it results in the immediate termination of a sustained tachycardia (termination of ventricular tachycardia by lidocaine or supraventricular tachycardia by adenosine or verapamil). The mechanism by which β-adrenergic antagonists decrease mortality after an acute myocardial infarction is not known.
B. The value of monitoring plasma drug concentrations in minimizing the risks associated with therapy is not established.
VIII. Antiarrhythmic Drug Pharmacology
A. Quinidine is a class IA drug that is effective in the treatment of acute and chronic supraventricular arrhythmia (rarely used because of its side effects). Supraventricular tachyarrhythmias associated with Wolff-Parkinson-White syndrome are effectively suppressed by quinidine.
1. Mechanism of Action. Quinidine decreases the slope of phase 4 depolarization, which explains its effectiveness in suppressing cardiac arrhythmias caused by enhanced automaticity.
2. Metabolism and Excretion
a. Quinidine is hydroxylated in the liver to inactive metabolites, which are excreted in the urine.
b. The concurrent administration of phenytoin or rifampin may lower blood levels of quinidine by enhancing liver clearance.
3. Side Effects. Quinidine has a low therapeutic ratio, with heart block, hypotension, and proarrhythmia being potential adverse side effects.
B. Procainamide is as effective as quinidine for the treatment of ventricular tachyarrhythmias but less effective in abolishing atrial tachyarrhythmias. Premature ventricular contractions and paroxysmal ventricular tachycardia are suppressed in most patients within a few minutes after intravenous (IV) administration, which is better tolerated than IV quinidine but may still cause hypotension.
1. Mechanism of Action
a. Procainamide is an analogue of the local anesthetic procaine.
b. Procainamide possesses an electrophysiologic action similar to that of quinidine but produces less prolongation of the QTc interval on the ECG (paradoxical ventricular tachycardia is a rare feature of procainamide therapy).
c. Procainamide has no vagolytic effect and can be used in patients with atrial fibrillation to suppress ventricular irritability without increasing the ventricular rate.
2. Metabolism and Excretion
a. Procainamide is eliminated by renal excretion and hepatic metabolism (dose of procainamide must be decreased when renal function is abnormal).
b. The activity of the N-acetyltransferase enzyme response for the acetylation of procainamide is genetically determined (in patients who are rapid acetylators, the elimination half-time of procainamide is 2.5 hours compared with 5 hours in slow acetylators).
3. Side Effects
a. Similar to quinidine, use of procainamide has dramatically decreased due to its side effect profile and availability of newer agents.
b. Hypotension that results from procainamide is more likely to be caused by direct myocardial depression than peripheral vasodilation.
c. Chronic administration of procainamide may be associated with a syndrome that resembles systemic lupus erythematosus.
C. Disopyramide is comparable to quinidine in effectively suppressing atrial and ventricular tachyarrhythmias. About 50% of the drug is excreted unchanged by the kidneys.
1. Side Effects
a. The most common side effects of disopyramide are dry mouth and urinary hesitancy, both of which are caused by the drug’s anticholinergic activity.
b. Prolongation of the QTc interval on the ECG and paradoxical ventricular tachycardia (similar to quinidine) may occur.
c. Disopyramide has significant myocardial depressant effects and can precipitate congestive heart failure and hypotension.
D. Moricizine is a phenothiazine derivative that is reserved for the treatment of life-threatening ventricular arrhythmias when other drugs such as amiodarone are not available or contraindicated (e.g., allergy).
1. Side Effects. Proarrhythmic effects occur in 3% to 15% of patients treated chronically with moricizine.
E. Lidocaine is used principally for suppression of ventricular arrhythmias, having minimal if any effect on supraventricular tachyarrhythmias. The efficacy of prophylactic lidocaine therapy for preventing early ventricular fibrillation after acute myocardial infarction has not been documented and is no longer recommended. Advantages of lidocaine over quinidine or procainamide are the more rapid onset and prompt disappearance of effects when the continuous infusion is terminated, greater therapeutic index, and a much reduced side effect profile. Lidocaine for IV administration differs from that used for local anesthesia because it does not contain a preservative.
1. Mechanism of Action
a. The effectiveness of lidocaine in suppressing premature ventricular contractions reflects its ability to decrease the rate of spontaneous phase 4 depolarization.
b. The ineffectiveness of lidocaine against supraventricular tachyarrhythmias presumably reflects its inability to alter the rate of spontaneous phase 4 depolarization in atrial cardiac cells.
2. Metabolism and Excretion. Lidocaine is metabolized in the liver, and resulting metabolites may possess cardiac antiarrhythmic activity.
3. Side Effects
a. Lidocaine is essentially devoid of effects on the ECG or cardiovascular system when the plasma concentration remains less than 5 μg/mL (does not alter the duration of the QRS complex on the ECG, and activity of the sympathetic nervous system is not changed).
b. Toxic plasma concentrations of lidocaine (>5 to 10 μg/mL) produce peripheral vasodilation and direct myocardial depression, resulting in hypotension.
c. Stimulation of the central nervous system (CNS) occurs in a dose-related manner, with symptoms appearing when plasma concentrations of lidocaine are greater than 5 μg/mL. Seizures are possible at plasma concentrations of 5 to 10 μg/mL.
d. CNS depression, apnea, and cardiac arrest are possible when plasma lidocaine concentrations are greater than 10 μg/mL.
e. The convulsive threshold for lidocaine is decreased during arterial hypoxemia, hyperkalemia, or acidosis (importance of monitoring these parameters during continuous infusion of lidocaine to patients for suppression of ventricular arrhythmias).
F. Mexiletine is an orally effective amine analogue of lidocaine that is used for the chronic suppression of ventricular cardiac tachyarrhythmias. As it is a lidocaine analog, mexiletine may be effective in decreasing neuropathic pain for patients in whom alternative pain medications have been unsatisfactory.
1. Side Effects
a. Neurologic side effects include tremulousness, diplopia, vertigo, and occasionally slurred speech.
b. Increases in liver enzymes may occur especially in patients manifesting congestive heart failure.
G. Tocainide is an orally effective amine analogue of lidocaine that is used for the chronic suppression of ventricular cardiac tachyarrhythmias.
H. Phenytoin is particularly effective in suppression of ventricular arrhythmias associated with digitalis toxicity and may be useful in the treatment of paradoxical ventricular tachycardia or torsades de pointes. The IV dose is 100 mg (1.5 mg/kg) every 5 minutes until the cardiac arrhythmia is controlled or 10 to 15 mg/kg (maximum 1,000 mg) has been administered. Because phenytoin can precipitate in 5% dextrose in water, it is preferable to give the drug via a delivery tubing containing normal saline.
1. Mechanism of Action
a. Phenytoin exerts a greater effect on the electrocardiographic QTc interval than does lidocaine and shortens the QTc interval more than any of the other antiarrhythmic drugs.
b. The ability of some volatile anesthetics to depress the sinoatrial node is a consideration if administration of phenytoin during general anesthesia is planned.
2. Metabolism and Excretion
a. Phenytoin is hydroxylated and then conjugated with glucuronic acid for excretion in the urine (impaired hepatic function may result in higher than normal blood levels of the drug).
b. Warfarin, phenylbutazone, and isoniazid may inhibit metabolism and increase phenytoin blood levels.
3. Side Effects
a. Phenytoin toxicity most commonly manifests as CNS disturbances, especially cerebellar disturbances (ataxia, nystagmus, vertigo, slurred speech, sedation, mental confusion).
b. Phenytoin partially inhibits insulin secretion and may lead to increased blood glucose levels in patients who are hyperglycemic.
c. Leukopenia, granulocytopenia, and thrombocytopenia may occur as a manifestation of drug-induced bone marrow depression.
I. Flecainide is a fluorinated local anesthetic analogue of procainamide that is more effective in suppressing ventricular premature beats and ventricular tachycardia than quinidine and disopyramide. Flecainide is also effective for the treatment of atrial tachyarrhythmias (effective for the treatment of tachyarrhythmias).
1. Metabolism and Excretion
a. Oral absorption of flecainide is excellent, and a prolonged elimination half-time (about 20 hours) makes a twice daily dose of 100 to 200 mg acceptable (not available in an IV formulation).
b. Elimination of flecainide is decreased in patients with congestive heart failure or renal failure and decreased left ventricular function.
2. Side Effects
a. Proarrhythmic effects occur in a significant number of treated patients especially in the presence of left ventricular dysfunction.
b. Flecainide prolongs the QRS complex and may depress sinoatrial node function as do β-adrenergic antagonists and calcium channel blockers (not administered to patients with second- and third-degree atrioventricular heart block).
c. The most common noncardiac adverse effect of flecainide is dose-related blurred vision.
d. Flecainide increases the capture thresholds of pacemakers.
J. Propafenone, like flecainide, is an effective oral antiarrhythmic drug for suppression of ventricular and atrial tachyarrhythmias. The rate of metabolism is genetically determined with about 90% of patients able to metabolize propafenone efficiently in the liver (availability of propafenone increases significantly in the presence of liver disease).
1. Side Effects
a. Propafenone depresses the myocardium and may cause conduction abnormalities such as sinoatrial node slowing, atrioventricular block, and bundle branch block.
b. Propafenone interferes with the metabolism of propranolol and metoprolol resulting in increased plasma concentrations of these β blockers. This drug also increases the plasma concentration of warfarin and may prolong the prothrombin time.
K. β-Adrenergic antagonists are effective for treatment of cardiac arrhythmias related to enhanced activity of the sympathetic nervous system (perioperative stress). Multifocal atrial tachycardia may respond to esmolol or metoprolol but is best treated with amiodarone. Acebutolol is effective in the treatment of frequent premature ventricular contractions. β-Adrenergic antagonists, especially propranolol, may be effective in controlling torsades de pointes for patients with prolonged QTc intervals. Acebutolol, propranolol, and metoprolol are approved for prevention of sudden death following myocardial infarction.
1. Mechanism of Action
a. The antiarrhythmic effects of β-adrenergic antagonists most likely reflect blockade of the responses of β receptors in the heart to sympathetic nervous system stimulation, as well as the effects of circulating catecholamines (rate of spontaneous phase 4 depolarization is decreased and the rate of sinoatrial node discharge is decreased).
b. β-Adrenergic antagonists can depress the myocardium not only by β blockade but also by direct depressant effects on cardiac muscle.
c. The usual oral dose of propranolol for chronic suppression of ventricular arrhythmias is 10 to 80 mg every 6 to 8 hours. Effective β blockade is usually achieved in an otherwise normal person when the resting heart rate is 55 to 60 beats per minute. For emergency suppression of cardiac arrhythmias in an adult, propranolol may be administered IV in a dose of 1 mg per minute (3 to 6 mg).
2. Metabolism and Excretion
a. Orally administered propranolol is extensively metabolized in the liver, and a hepatic first-pass effect is responsible for the variation in plasma concentration.
b. Propranolol readily crosses the blood–brain barrier.
c. The principal metabolite of propranolol is 4-hydroxypropranolol, which possesses weak β-adrenergic antagonist activity.
3. Side Effects
a. Bradycardia, hypotension, myocardial depression, and bronchospasm are side effects of β-adrenergic antagonists that reflect the ability of these drugs to inhibit sympathetic nervous system activity. The use of propranolol in patients with preexisting atrioventricular heart block is not recommended.
b. Interference with glucose metabolism may manifest as hypoglycemia in patients being treated for diabetes mellitus.
c. Upregulation of β-adrenergic receptors occurs with chronic administration of β-adrenergic antagonists such that abrupt discontinuation of treatment may lead to supraventricular tachycardia.
L. Amiodarone is a potent antiarrhythmic drug with a wide spectrum of activity against refractory supraventricular and ventricular tachyarrhythmias. In the presence of ventricular tachycardia or fibrillation that is resistant to electrical defibrillation, amiodarone 300 mg IV is recommended. Preoperative oral administration of amiodarone decreases the incidence of atrial fibrillation after cardiac surgery. It is also effective for suppression of tachyarrhythmias associated with Wolff-Parkinson-White syndrome. Similar to β blockers and unlike class I drugs, amiodarone decreases mortality after myocardial infarction. After initiation of oral therapy, a decrease in ventricular tachyarrhythmias occurs within 72 hours. After discontinuation of chronic oral therapy, the pharmacologic effect of amiodarone lasts for a prolonged period (up to 60 days), reflecting the prolonged elimination half-time of this drug (Fig. 21-2).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


