http://evolve.elsevier.com/Edmunds/NP/
Antiarrhythmics are used for the treatment of fast, slow, or irregular heartbeat. Most drugs used as antiarrhythmics in primary care are used for other indications and are discussed in detail in other chapters. Because amiodarone and dronedarone are the only drugs that are used exclusively for arrhythmia, they are the only drugs discussed here in detail. The primary care provider who has a patient taking an antiarrhythmic should consult the most recent sources for complete, up-to-date information on the drug and should work closely with a cardiologist, electrophysiologist, or experienced physician. All antiarrhythmic medications have potentially serious adverse effects, including the induction of other serious and even fatal arrhythmias.
Therapeutic Overview of Arrhythmias
Only the three arrhythmias most commonly managed in the outpatient setting are discussed in detail: paroxysmal supraventricular tachycardia (PSVT), atrial fibrillation (AF), and premature ventricular contractions (PVCs).
Anatomy and Physiology
The heart is composed of specialized myocardial muscle cells that have the capacity to generate an electrical potential (automaticity) and spread the electrical current from cell to cell (conductivity) (Figure 23-1). Many cells within the myocardium can serve as a pacemaker of the heart, but the primary pacemaker is found in the sinoatrial node. Electrical waves of depolarization spread through the atrium, the atrio-ventricular junction, and the bundle of His, and down the left- and right-bundle branches, producing synchronized atrial and ventricular muscular contractions. When the dominant pacemaker slows or does not fire, other cells take over and continue the heartbeat, although at a slower rate. Sometimes aberrant cells take over the pacemaker role, creating irregular rhythms and/or tachyarrhythmias.
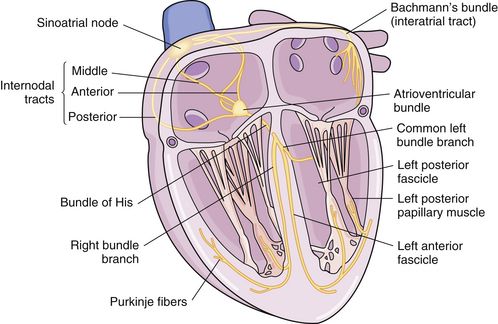
FIGURE 23-1 Conduction system of the heart. (From Monahan, Sands, Neighbors, Marek, & Green: Phipp’s Medical-Surgical Nursing: Health and Illness Perspectives, ed 8, St. Louis, 2006, Mosby.)
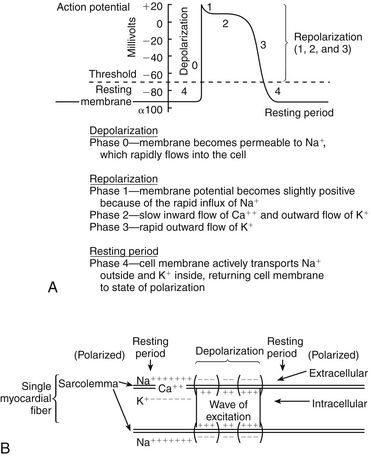
Action potential (Figure 23-2) refers to the difference in electrical charge across the myocardial cell membrane that results in polarization and depolarization. Depolarization is the electrical impulse that precedes the mechanical contraction of cardiac tissue. Repolarization is the recovery stage after muscle contraction.
Pathophysiology
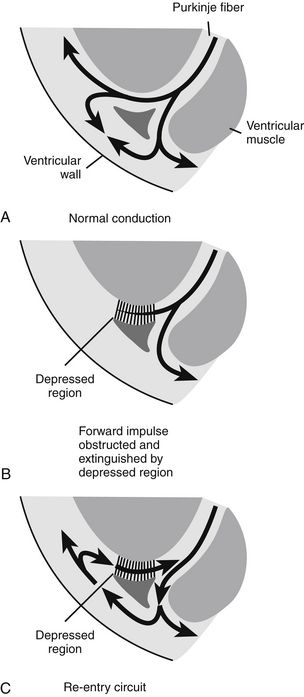
The mechanism of an arrhythmia is theoretically important in determining which drug will be effective. The two basic tachyarrhythmic mechanisms within the heart are (1) increased automaticity, resulting in an ectopic focus, and (2) reentry through abnormal conduction pathways. However, it is often clinically impossible to determine the mechanism without an electrophysiology mapping study. Arrhythmias that are caused by irritability or increased automaticity are treated with drugs that prolong the action potential, thus decreasing the rate at which impulses can be generated (Figure 23-3). Sustained ventricular tachycardia usually occurs as reentry, and it is treated with a drug that prolongs the effective refractory period.
Disease Process
PSVT is a term that applies to all supraventricular tachycardias except for AF and atrial flutter. In approximately 60% of cases, it is due to reentry within or in close proximity to the AV node known as atrioventricular nodal reentrant tachycardia (AVNRT). It results from atrioventricular reentrant tachycardia (AVRT) in 30% of cases. AVRT refers to accessory pathways that connect the atrium with the ventricle. It is characterized by a heart rate of 130 to 250, 1:1 conduction, and a narrow QRS complex. PSVTs often are seen in patients with no underlying heart disease. Treatment depends on the patient’s ability to tolerate the tachycardia, the mechanism of the arrhythmia, and how frequently the arrhythmia presents.
AF is a relatively common arrhythmia. Risk factors include male gender, underlying cardiovascular disease, surgery, emotional stress, and increasing age, and use of painkillers such as NSAIDs and, COX-2 inhibitors. It is due to a total lack of organized electrical activity in the atria. AF is classified according to the duration of the arrhythmia. Paroxysmal AF refers to episodes that self-terminate and last less than 7 days—usually less than 24 hours. Persistent AF lasts longer than 7 days, and episodes fail to terminate on their own. However, they can be terminated by electrical cardioversion after absence of clots in the left atrium has been confirmed by transesophageal echocardiogram and therapeutic warfarin therapy for 3 weeks and initiation of amiodarone. Permanent AF lasts for longer than 1 year, and termination has not been attempted or has failed. Finally, “lone” AF is applied to any form of AF that occurs in patients with no history of structural cardiac disease.
Treatment of patients with AF focuses on rate and rhythm control and anticoagulation to prevent systemic embolization. Rate control with β-blockers, calcium channel blockers, or digoxin and anticoagulation with warfarin is recommended for most patients with AF. Rhythm control is attained through the use of DC or medications. Long-term maintenance of normal sinus rhythm is not usually successful over time if the patient has a history of valvular disease or longstanding hypertension. AF can be classified as paroxysmal, persistent, or chronic and is associated with two problems. The first is rapid ventricular response. The ventricular response usually occurs at a rate of 150 to 220 beats/min. This rapid rate may be poorly tolerated, precipitating CHF if the patient cannot tolerate loss of atrial kick. The second issue with AF is the risk of thromboembolism. Clots may form in the atria as the result of pooling of blood that occurs with chaotic firing and lack of contractility; they can be released into the systemic circulation, causing arterial occlusion and stroke.
PVCs are caused by increased automaticity. They are classified according to both prevalence and morphologic characteristics. The most important factor in determining whether to treat PVCs is the presence of underlying heart disease. Underlying heart disease (especially myocardial ischemia or recent MI) in the presence of PVCs can be a marker for development of malignant ventricular arrhythmias. Additional factors that place the patient at increased risk are the presence of cardiac scarring, hypertrophy, and/or left ventricular dysfunction. PVCs that are frequent, paired, or sustained are particularly dangerous. PVCs that occur during the QT interval present a risk for initiating ventricular fibrillation. Because of the risks inherent in antiarrhythmic therapy, patients should not be treated unless clearly indicated.
Torsades de pointes is a life-threatening ventricular tachycardia that is associated with prolongation of the QT interval. There is recognition that Long QT is also a separate syndrome that arises, usually from a genetic defect. In many cases, drugs may precipitate sudden acute cardiac arrest in individuals who have the genetic tendency to long QT syndrome (LQTS). So using some drugs to protect these individuals, as well as avoiding drugs that may precipitate the event, is lifesaving once the patient is diagnosed with this genetic mutation.
Assessment
Take a thorough health history, including history of drug hypersensitivity and review of other medications that may cause drug interactions, other medical comorbidities, and current antiarrhythmic agents that could cause an additive cardiac depressant effect. The patient with an arrhythmia may have no complaints or may complain of chest pain, shortness of breath, fatigue, palpitations, dizziness, weakness, or syncope. The palpitations may be described as fluttering, pounding, skipped beats, or “heart jumping out of the chest.” These often are noticed when the patient is sitting quietly or lying in bed.
Auscultation often gives clues as to the rate and rhythm of the heart. AF is recognized by an irregular rhythm and rate, whereas PSVT is a very fast regular rate and rhythm. PVCs are premature ventricular beats with a compensatory pause. Premature atrial contractions (PACs) occur early and cause an occasional skip, with no compensatory pause.
Obtain relevant laboratory studies, such as chest radiograph, graded exercise test, ECG, echocardiogram, and 24-hour Holter monitor results. Obtain baseline laboratory values such as CBC, electrolytes, thyroid panel, and renal and hepatic function tests.
Identify and remove, or correct, any precipitating factors. Treat any underlying factors that may cause arrhythmia, such as hypoxia, acid-base imbalance, increased or decreased potassium or magnesium, thyroid dysfunction, excessive catecholamines, or drug toxicity. Anxiety, caffeine, cigarettes, or stimulants such as decongestants, diet pills, amphetamines, theophylline, nicotinic acid, or alcohol also may precipitate arrhythmia.
Mechanism of Action
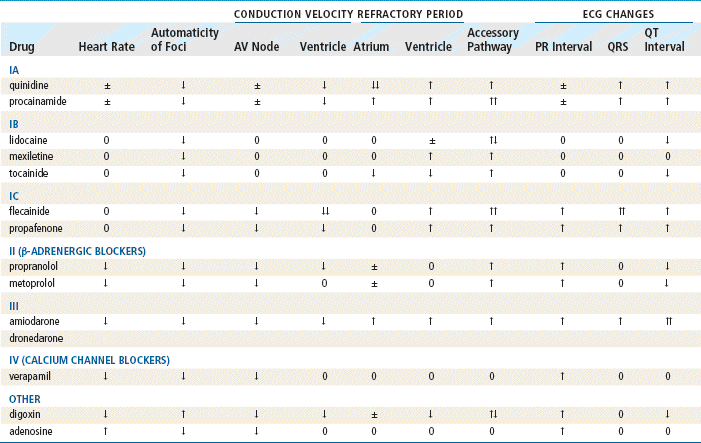
Antiarrhythmic drugs act to reduce electrical irregularity of the heart. They do this by altering the action potential of cardiac cells (Figure 23-4, Table 23-2). All antiarrhythmics have the potential to cause arrhythmia. In 1995, CAST (Cardiac Arrhythmia Suppression Trial) revealed the dangers of aggressive medical treatment of arrhythmias.
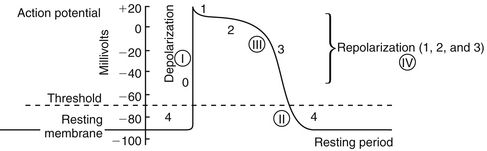
FIGURE 23-4 Phases of the action potential and locations of action of the classes of antiarrhythmics. Circled Roman numerals indicate the major site of action of that category of antiarrhythmic.
Class IA drugs (quinidine, disopyramide, procainamide) depress rapid depolarization (phase 0) of the action potential. These drugs act to slow conduction by lengthening the effective refractory period of atrial and ventricular myocardium, by depressing the inward sodium current, and by decreasing the automaticity and excitability of ectopic foci of cardiac muscle. Class IB drugs (lidocaine, mexiletine, tocainide) exert less effect on sodium channels at rest but are more prominent during depolarization, so their effect on phase 0 is only slight. Class IC drugs (flecainide, propafenone) depress phase 0 markedly and profoundly slow conduction.
β-Blockers are Class II antiarrhythmics that work by inhibiting sympathetic stimulation. They antagonize the effects of catecholamines released from the adrenergic nerve endings and the adrenal medulla. Blocking sympathetic activity reduces the rate of discharge of the sinus and other foci that act as pacemakers, and it increases the effective refractory period of the AV node.
Class III drugs (amiodarone, dronedarone, ibutilide, dofetilide, sotalol) prolong phase 3 repolarization by blocking potassium channels. The QT interval is thus prolonged on the ECG. This prolongation also increases the risk of torsades de pointes, a ventricular tachycardia. Amiodarone, however, does not appear to have this effect. Amiodarone blocks sodium channels, and this contributes to slowing of conduction and prolongs refractoriness in the AV node. Its vasodilatory action decreases cardiac workload and therefore decreases myocardial oxygen consumption. Dronedarone is a benzofuran derivative of amiodarone that was recently FDA approved. A high mortality rate in patients with atrial fibrillation taking this drug makes it questionable whether it will remain on the market. There is some indication it prolongs the QT interval. Sotalol exhibits β-blocking activity. Ibutilide is available only as an IV formulation and is used for rapid termination of atrial fibrillation and flutter. Dofetilide must be initiated in a hospitalized patient because of its ability to prolong the QT interval.
Class IV drugs are the nondihydropyridine calcium channel blockers (verapamil, diltiazem). These drugs inhibit calcium ion influx through slow channels into conductile and contractile myocardial cells and vascular smooth muscle cells; they also slow AV conduction and prolong the effective refractory period within the AV node.
Digoxin suppresses AV node conduction to increase the effective refractory period and decrease conduction. This results in a decreased heart rate and reduced risk of reentry or supraventricular arrhythmia.
Long QT syndrome is a unique physiologic condition where medications are often the cause and not the treatment of the problem. LQTS is a channelopathy characterized by prolongation of the QT interval on the electrocardiogram. Time is measured from the beginning of the QRS complex to the end of the T wave, and intervals longer than 0.44 seconds are generally considered abnormal; the problem is usually best seen in the right precordial leads. The QT interval represents the duration of activation and recovery of ventricular myocardial activity. Prolonged recovery from electrical excitation increases the likelihood of dispersing the recovery refractoriness, leaving some parts of the myocardium refractory to subsequent depolarization.
The clinical consequence of delayed repolarization may be polymorphic ventricular tachycardia, or torsades de pointes, which may lead to ventricular fibrillation and sudden cardiac death. This arrhythmia may result from physiologic changes that cause reactivation of calcium channels, reactivation of a delayed sodium current, or a decreased outward potassium current that results in early afterdepolarization. The LQT cardiac repolarization defect is often caused by a variety of genetic mutations that may be present congenitally or are acquired. The LQT interval may be lengthened by different factors in different people—for example, by exercise, drugs, or a startle reflex. It may be that all individuals with this problem have an underlying genetic defect and that medications only reveal the syndrome. Current research is examining whether many sudden infant death syndrome (SIDS) deaths or sudden acute cardiac deaths in youth athletes are related to LQTS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


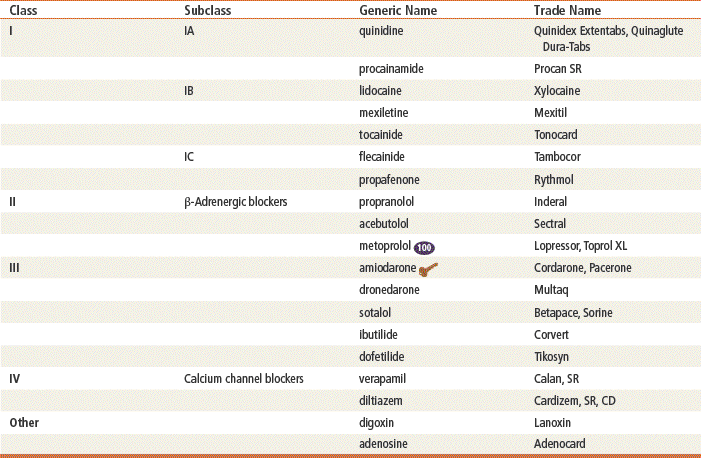
 Top 100 drug;
Top 100 drug;  key drug.
key drug.