Brian D. Campbell
Anesthesia
The first medical report of anesthesia was announced to the world on November 18, 1846, by Henry J. Bigelow in the Boston Medical and Surgical Journal. An era had ended during which successful surgery depended largely on the surgeon’s speed while working on a struggling, distressed patient. Anesthetic techniques gave the surgeon more time to operate and permitted new procedures to be undertaken that would have been impossible before. Many modern surgical techniques since have become feasible because advances in the art and science of anesthesia have occurred.
The word anesthesia is derived from the Greek word anaisthesia, which literally means “no sensation.” Anesthesia was listed in Bailey’s English Dictionary in 1721. When the effects of ether were discovered, Oliver Wendell Holmes suggested anesthesia be used as a name for the new phenomenon. Some believed that he coined this term; others believed that he knew of the Greek word that Plato had used. In any case, anesthesia was, in the memorable phrase of S. Weir Mitchell, the “death of pain.” From these early beginnings anesthesia has developed into a sophisticated science and “clinical art” that interfaces with many other medical specialties.
Without anesthesia most modern surgical procedures would not be feasible. As integral members of the patient care team in operative and other invasive procedure settings, perioperative nurses need to be familiar with the principles and practices of anesthesia and the perioperative functions of the anesthesia provider. This chapter presents an overview of the modern practice of anesthesia, the factors involved, and the interrelationship with the perioperative nurse. It discusses major types of anesthesia, introduces the more commonly used modern medications, reviews standards of anesthesia care, and summarizes problems that can occur during the perioperative period. The anesthesia machine and monitoring equipment also are described so that perioperative nurses can become familiar with their basic functions because the nurse may use them during local anesthesia or conscious sedation/analgesia procedures.
The sections of this chapter are organized so that they can be referred to independently without reading the entire chapter. Experienced perioperative nurses are familiar with the commonly used abbreviations used in this chapter. To provide a single reference source for the student or novice perioperative nurse, most abbreviations are defined in Box 5-1.
Anesthesia Providers
In the United States anesthesia care usually is provided in one of three ways: (1) by an anesthesiologist; (2) by a certified registered nurse anesthetist (CRNA) working alone, in collaboration with, or under the direction of an anesthesiologist or a physician; or (3) by an anesthesiologist’s assistant (AA) working under direct supervision of an anesthesiologist.
An anesthesiologist is a licensed physician with 4 or more years of specialty training in anesthesiology. Nurse anesthesia programs last a minimum of 2 years and require a bachelor of science (BS) degree in nursing or other appropriate field and a minimum of 1 year of critical care experience before acceptance. All nurse anesthesia programs are at the master’s degree level at a minimum. On completion, graduates must successfully complete a national certification examination. Several programs have already advanced to the doctoral level and by 2025, it is projected that all CRNAs will graduate with a doctor of nursing practice (DNP) degree.
Since 1969 AAs also have been used as assistants to anesthesiologists. Acceptance into an AA program requires a BS degree that includes college-level “premed” education. AAs are graduate students within a medical school and typically receive a master of medical science (MMS) degree from the medical school. They also take a national certification examination administered by the National Commission on Certification of Anesthesia Providers’ Assistants under the supervision of the National Board of Medical Examiners.
In this chapter the term anesthesia provider denotes the individual providing the continuous anesthesia care for the patient. Depending on the practice in a given hospital or surgical setting, this may be an anesthesiologist, a CRNA, or an AA. In many settings an anesthesia care team includes CRNAs, with or without AAs supervised by anesthesiologists. In small rural hospitals in many states, an anesthesiologist may not be present, and a CRNA may be the sole anesthesia provider.
The anesthesia provider is the patient’s advocate in the perioperative period; as such, he or she must be concerned with many divergent factors when the patient’s own sensory and cerebral functions are obtunded by anesthesia. The field of anesthesia has become so complex that in many large hospitals an anesthesia provider may specialize further in obstetric, neurosurgical, pediatric, cardiovascular, regional, or ambulatory anesthesia. The anesthesia provider also may subspecialize in acute and chronic pain management or in critical care medicine.
Patient Safety
Patient safety is always a primary concern during surgery and anesthesia. About 32 million anesthetics are administered each year in the United States. Of these, data from several sources indicate a death rate ranging from 1 per 35,000 to about 1 per 40,000. These rates represent a significant decline during the past 30 years, despite surgical procedures being performed on increasingly higher risk patients than in the past. A study from Germany showed an increase in death rates within 1 year post general anesthesia and as high as 1 in 10 for the population age 65 or more. The authors concede that the anesthesia death rate has not declined, but patients are now older and sicker (Gottschalk et al, 2011).
The general public still considers anesthesia to be a major risk of surgery. This attitude may be attributable to sensationalized reports in the news media, magazine articles, and in movies. In addition, people may have a heightened awareness of anesthesia-related deaths because these often occur acutely in the perioperative period, whereas surgical or medical problems unrelated to anesthesia may not result in death until days after the procedure.
Environmental Noise
Many studies demonstrate the effects that noise has on humans. Multiple conversations, loud music, and other noises can create or worsen patient anxiety, as well as make communication difficult among team members and between the patient and team members. Noise can also cause distraction, increasing the potential for miscommunication and errors (AORN, 2013). Patients recovering during the immediate postoperative period have expressed dissatisfaction with high noise levels and overhearing staff member conversations. Improvement in patient satisfaction can be achieved when the noise level lessens in the postanesthesia care unit (PACU) (Smykowski, 2008). While the patient is in the operating room (OR), especially during induction and emergence, every effort should be made to maintain a calm, quiet environment. This is especially true when caring for the pediatric patient. Hearing multiple voices can create patient anxiety and confusion.
Awareness During Anesthesia
Remaining conscious during anesthesia is a concern of both patients and anesthesia providers. Some patients are so anxious about being aware of anything during surgery that it may affect their reasoning when discussing the options for anesthesia. Many procedures, such as biopsies, inguinal hernias, or procedures on the lower extremities, can be done under regional anesthesia or monitored anesthesia care (MAC). These patients may want general anesthesia, however, because they do not want to be aware of anything during the procedure. In rare cases, during general anesthesia for emergent procedures or trauma, the patient may be paralyzed, aware of what is occurring but unable to tell anyone.
Intraoperative awareness (IOA) is reported with multiple and differing anesthetic techniques. Several factors may contribute to its occurrence. Overall, 20,000 to 40,000 patients each year experience awareness during general anesthesia (Bazzell et al, 2008). The incidence of awareness may increase to 1% to 1.5% in higher risk patient populations, such as patients requiring anesthesia for obstetrics, major trauma, and cardiac surgery (Ghoneim et al, 2009).
For many years, the electroencephalogram (EEG) was the standard for assessing a person’s hypnotic and sleep state. However, using an EEG for all surgical procedures is unrealistic. The late 1990s saw development of the bispectral index system (BIS), which analyzes the relationship and frequency of brain signals using an algorithm to generate a composite, numeric value that seems to correlate with the cerebral state. Four electrodes, positioned across the forehead, connect to a monitor that gives an index (0 to 100) of the patient’s hypnotic state or sedation level; an index of 40 to 60 is considered optimal anesthesia. The system monitors the effects of anesthetics and sedatives on the hypnotic status of the brain, but is less informative about the level of analgesia. Motion artifacts and mental changes cause erratic changes under lighter levels of sedation commonly used with MAC. The BIS monitor is not a predictor of motor depression; it also lags by 3 to 5 seconds, leading to a potential for the anesthesia provider not anticipating a sudden rise in the depth of anesthesia. Nonetheless, use of the BIS monitor to alert the anesthesia provider to intraoperative awareness with recall (AWR) using a BIS-based protocol can be effective (Avidan and Mashour, 2013).
Preoperative Preparation
Patient Evaluation
Preoperative evaluation is done in advance of the scheduled surgical procedure, often 1 or more days before the procedure, in a preadmission clinic (sometimes called preadmission testing, preanesthesia clinic, or anesthesia-assessment unit). Preadmission staff secure admission data, appropriate consent forms, and a preoperative history; they also perform a physical examination, complete a preanesthesia evaluation and examination, obtain an airway history and patient’s weight, and process appropriate diagnostic or laboratory tests. Numerous studies have shown only limited benefits from extensive routine preoperative testing (Chung et al, 2009). Patients with higher than average risk based on history are those who usually require more extensive testing. Preoperative care based on careful preanesthesia evaluation can result in significant reduction in costs (Issa, 2011). After assessing the patient’s physical status, the anesthesia provider in the preadmission setting selects the most appropriate anesthetic technique. Resolving the patient’s questions and concerns follows, as well as instructions aimed to expedite admission on the day of surgery. Prior to elective surgery, the patient should be in optimal medical condition. A primary benefit of preoperative testing is to identify patients at risk for perioperative complications so that appropriate perioperative therapy can foster a return to functional status. Morbidly obese patients present technical and clinical challenges during anesthesia management (Ortiz and Wiener-Kronish, 2010).
Anesthesia, even in healthy patients, presents particular risks. Risk associated with anesthesia is unique in that rarely does the anesthetic itself offer benefit; rather, it allows performing surgical interventions and procedures that may benefit the patient enormously. Another goal in risk assessment is to inform patients so that they can weigh options and identify opportunities to alter that risk. Analysis of the first 2000 reports submitted in the Australian Incident Monitoring Study (AIMS) found a sixfold increase in mortality in patients who were inadequately assessed preoperatively. In a different study of anesthetic-related perioperative deaths, nearly 40% of deaths involved inadequate preoperative assessment and management (Miller, 2010). If it is determined that the patient’s physical status should improve so as to reduce the risks involved, the patient’s primary physician or surgeon discusses this with the patient, and, if necessary, elective surgery is deferred until the patient’s condition optimizes. In emergent surgery, however, any benefits gained from a delay must be weighed carefully against the hazards of deferral.
The assignment of a physical status classification depends on the patient’s physiologic condition independent of the proposed surgical procedure. The physical status classification was developed by the American Society of Anesthesiologists (ASA) to provide uniform guidelines. It is an evaluation of the severity of systemic diseases, physiologic dysfunction, and anatomic abnormalities. The ASA classification system is widely used to estimate perioperative risk (Table 5-1).
TABLE 5-1
ASA Physical (P) Status Classification
| Status*† | Definition | Description and Examples |
| P1 | Normal healthy patient | No physiologic, psychologic, biochemical, or organic disturbance |
| P2 | Patient with mild systemic disease | Cardiovascular disease with minimal restriction of activity; hypertension, asthma, chronic bronchitis, obesity, diabetes mellitus, or tobacco abuse; mild asthma or well-controlled hypertension. No significant impact on daily activity. Unlikely impact on anesthesia and surgery. |
| P3 | Patient with a severe systemic disease that limits activity, but is not incapacitating | Cardiovascular or pulmonary disease that limits activity; severe diabetes with systemic complications; history of myocardial infarction, angina pectoris, poorly controlled hypertension, or morbid obesity; renal failure on dialysis or class 2 congestive heart failure. Significant impact on daily activity. Likely impact on anesthesia and surgery |
| P4 | Patient with a severe systemic disease that is a constant threat to life or requires intensive therapy | Severe cardiac, pulmonary, renal, hepatic, or endocrine dysfunction, acute myocardial infarction, respiratory failure requiring mechanical ventilation. Serious limitation of daily activity. Major impact on anesthesia and surgery |
| P5 | Moribund patient who is not expected to survive 24 hr with or without operation | Surgery is done as last recourse or resuscitative effort; major multisystem or cerebral trauma, ruptured aneurysm, or large pulmonary embolus |
| P6 | Patient declared brain dead whose organs are being removed for donor purposes |
*In statuses 2, 3, and 4, the systemic disease may or may not be related to the reason for surgery.
†For any patient (P1 through P5) requiring emergency surgery, an E is added to the physical status, such as P1E, P2E. ASA 1 through ASA 6 or I to VI is often used for physical status.
Modified from American Society of Anesthesiologists (ASA): Manual for anesthesia departments, Park Ridge, IL, 1997, The Association, available at www.asahq.org/Home/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System. Accessed February 3, 2013.
Although many hospitals and ambulatory surgery centers commonly use preadmission clinics, nurses may conduct preoperative telephone interviews with patients in reasonably good health. Such nurses pose questions relating to pulmonary and cardiac disease; medication (prescription, over-the-counter, herbal and homeopathic remedies) and alcohol use; medication, latex, or anesthetic allergies; pregnancy; and personal or family history of anesthetic reactions. The preadmission interview, whether by phone or in person, provides an opportunity for patient education as it relates to the proposed procedure (Patient and Family Education).
On the day of surgery, patients arrive 1 to 2 hours before the scheduled surgery to complete other preoperative processes. In some facilities certain ambulatory patients are evaluated further just before surgery. These are usually healthy patients having minor procedures or patients with stable, chronic conditions about to undergo a procedure (e.g., cataract removal, skin lesion excision) under MAC. These preadmission processes reduce costs of healthcare, decrease risk of healthcare-associated infections associated with longer hospital admissions, increase use and efficiency of healthcare resources, improve patient relations, and enhance the chances of having a well-informed patient in optimal health status both before and after the proposed procedure.
In larger hospitals and ambulatory surgery centers the patient evaluator in the preanesthesia clinic is often not the anesthesia provider for the patient’s surgical procedure. Immediately before surgery, therefore, the anesthesia provider (1) reviews the patient’s chart, laboratory data, and diagnostic studies, such as electrocardiogram (ECG) and chest x-ray if ordered and necessary; (2) confirms that the appropriate consent forms (surgery, anesthesia, use of blood products) have been signed; (3) identifies the patient; (4) verifies the surgical procedure; (5) reviews the choice of anesthesia; (6) examines the patient; and (7) administers preoperative medications as indicated (Miller, 2010). As ambulatory surgery becomes increasingly common, other factors are taken into consideration (Ambulatory Surgery Considerations).
Choice of Anesthesia
The patient, anesthesia provider, and surgeon make the choice of anesthesia for a given surgical procedure. Many factors influence this choice, including the following:
1. Patient’s wishes and understanding of the types of anesthesia that could be used
2. Patient’s physiologic status
3. Presence and severity of coexisting diseases
4. Patient’s mental and psychologic status
5. Postoperative recovery from various kinds of anesthesia
6. Options for management of postoperative pain
7. Type and duration of surgical procedure
8. Patient’s position during surgery
Premedication
The primary purpose of premedication before anesthesia is to sedate the patient and reduce anxiety. Medications that may be given preoperatively include sedatives and hypnotics, anxiolytics, amnestics, tranquilizers, narcotics or other analgesics, antiemetics, and anticholinergics. A single medication may possess the properties of several medication classes. Midazolam (Versed) is administered frequently to relieve apprehension and to provide amnesia. An analgesic or narcotic may be ordered if preoperative discomfort is anticipated during invasive procedures or during the administration of a regional anesthetic. An anticholinergic, such as atropine or glycopyrrolate, may be used to prevent bradycardia in pediatric patients, to control secretions in patients undergoing oropharyngeal procedures, or to control cardiac reflex that may cause bradycardia (e.g., during ophthalmic procedures) (Miller, 2010).
To decrease the risk of aspiration, metoclopramide (Reglan) may be given to empty the stomach and to reduce nausea and vomiting. In addition, an antacid or an H2-receptor–blocking medication, such as cimetidine (Tagamet), ranitidine (Zantac), or famotidine (Pepcid), may be included to decrease gastric acid production or the acidity of the gastric contents, or both. Chemoprophylaxis, with medications such as these, is part of safe airway management.
Before administering premedication, the anesthesia team answers any last-minute questions from the patient concerning surgery and anesthesia, and completes the preoperative verification process, or “anesthesia time-out,” to ensure that all relevant documents (e.g., the history and physical examination, surgical consent) and imaging studies (properly labeled and displayed) are available before the start of the procedure. The anesthesia team reviews these documents, which must be consistent with the patient’s stated expectations (when the patient is awake and aware, the patient should actively participate in the verification process). The surgical team must agree that this is the correct patient and the correct procedure on the correct side and site. Any additional special equipment, supplies, or implants also are confirmed as correct and available. Team members must also mark the surgical site before administering premedication.
Administration of premedication(s) may be intramuscular (IM), intravenous (IV), intranasal, or oral (PO) with 15 to 30 mL of water. Patients usually prefer oral premedication, and the small amount of water is readily absorbed directly across the gastric mucosa. Except for the small amount of water needed to swallow any medications, adult patients traditionally must maintain a nothing-by-mouth (NPO) status for a minimum of 4 to 6 hours before elective surgery. More recent data suggest, however, that clear liquids are acceptable up to 2 hours before surgery (ASA, 2011) (Table 5-2). Alternatively, IV premedication is administered 30 to 90 minutes before surgery in preoperative holding or after the patient arrives in the surgical suite. Medication shortages in the United States have had an effect on the preoperative medication chosen (Research Highlight).
Table 5-2
| Ingested Material Allowed | Minimal Fasting Period Recommended |
| Food and fluids as desired | Up to 8 hours |
| Light meal (e.g., toast and clear liquids, infant formula, and non-human milk) | Up to 6 hours |
| Breast milk | Up to 4 hours |
| Clear liquids only, NO solid food or foods with fat content† | Up to 2 hours |
| No solids or liquids | During the 2 hours until surgical time |
*These guidelines are recommended for healthy patients undergoing elective procedures. They are not intended for women in labor. They do not guarantee complete gastric emptying. The fasting periods noted above apply to patients of all ages.
†Clear liquids include water, fruit juices without pulp, black coffee, clear tea, and carbonated beverages. Patients should also be instructed not to chew gum or eat any candies or mints. The type of fluid is more important than the amount and should never include alcohol.
Modified from The American Society of Anesthesiologists: Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures, an updated report, Anesthesiology 114(3):495–511, 2011.
Although premedication use is common, studies have shown that visits before surgery by the anesthesia provider and the perioperative nurse are similarly important to relieve patient anxiety and concern. Major patient concerns include fear of the unknown, relinquishing control of one’s life to someone else, being awake during surgery, and not awakening from anesthesia, as well as concerns related to the surgery itself (e.g., diagnosis, prognosis). Premedication may be unnecessary for older patients because their anxiety levels are lower, their responses to medications are unpredictable, and IV sedation can be given in the OR if required.
Types of Anesthesia Care
TJC has issued its Comprehensive Accreditation Manual for Hospitals, which includes anesthesia care standards that apply when patients receive, in any setting, moderate or deep sedation or general, spinal, or other major regional anesthesia. Descriptions of frequently used classifications of anesthesia care follow.
General Anesthesia
General anesthesia is a reversible, unconscious state characterized by amnesia (sleep, hypnosis, or basal narcosis), analgesia (freedom from pain), depression of reflexes, muscle relaxation, and homeostasis or specific manipulation of physiologic systems and functions. Most patients think of general anesthesia when they are scheduled to have a surgical procedure; that is, they expect to be “put to sleep.” As such, they experience a medication-induced loss of consciousness during which they are not arousable. Their ability to maintain ventilatory function is often impaired, requiring assistance maintaining a patent airway. Positive-pressure ventilation may be required, given the possibility of decreased spontaneous ventilation or medication-induced depression of neuromuscular function.
Regional Anesthesia
Regional anesthesia is defined broadly as a reversible loss of sensation in a specific area or region of the body when a local anesthetic is injected to block or anesthetize nerve fibers in and around the operative site. Common regional anesthesia includes spinal (also called “subarachnoid block” or SAB), epidural, caudal, and major peripheral nerve blocks. Although ultrasound guidance has been reviewed as a technique to enhance regional anesthesia and analgesia, it may not eliminate complications such as blood vessel puncture or inadvertent intraneural or intravascular injections. Nonetheless, in some healthcare facilities, ultrasound-guided regional anesthesia and analgesia is used to reduce time or number of attempts to perform blocks (Liu et al, 2009).
Monitored Anesthesia Care
MAC is infiltration of the surgical site with a local anesthetic and is performed by the surgeon (note that “local standby” and “anesthesia standby” are older, less accurate terms frequently used interchangeably with MAC). The anesthesia provider then supplements the local anesthesia with IV medications that provide sedation and systemic analgesia, monitors the patient’s vital functions, and may use additional medication to optimize the patient’s physiologic status. MAC is often used for healthy patients undergoing relatively minor surgical procedures. It also may be used for some procedures for critically ill patients who may tolerate a general anesthetic poorly.
Moderate Sedation/Analgesia
Moderate sedation/analgesia (conscious sedation) is administered for specific short-term surgical, diagnostic, and therapeutic procedures performed within a hospital or ambulatory center. As early as 2003, the ASA updated its Practice Guidelines for Sedation and Analgesia by Non-Anesthesiologists. The Association of periOperative Registered Nurses (AORN) went on to define moderate sedation/analgesia as “a drug-induced, mild depression of consciousness achieved by the administration of sedatives or the combination of sedatives and analgesic medications, most often administered intravenously, and titrated to achieve a desired effect” (2013, p. 411). Patients can maintain a patent airway and require no interventions; spontaneous ventilation remains adequate. Cardiovascular function is usually maintained. Patients whose only response is reflex withdrawal from a painful stimulus are sedated to a greater degree than that encompassed by conscious sedation/analgesia.
The demand for appropriate providers to administer and monitor the patient receiving conscious sedation/analgesia has grown and now exceeds the supply of anesthesia providers. This demand has resulted in increased use of professional registered nurses with additional training in administering moderate sedation/analgesia medications and monitoring these patients. Various medications and techniques are used to achieve conscious sedation/analgesia, each with advantages and disadvantages. Competency-based education programs and assessment should be established for nurse-monitored sedation. AORN publishes recommendations for managing patients undergoing moderate sedation/analgesia that should be used by healthcare facilities to develop such programs (AORN, 2013).
Local Anesthesia
Local anesthesia refers to the administration of an anesthetic agent to one part of the body by local infiltration or topical application, usually administered by the surgeon. Local anesthesia is used (1) for minor procedures, (2) if the patient’s cooperation is necessary for the procedure, or (3) if the patient’s physical condition warrants its use. An anesthesia provider is not involved in the patient’s care. A perioperative nurse monitors the patient’s vital signs, observes for symptoms of toxicity (Wolfe, 2011), and provides supportive care during the procedure. AORN (2013) recommendations for managing patients undergoing local anesthesia and documenting patient care should be used to establish policies and procedures in operative and other invasive settings.
Perioperative Monitoring
Significant advances continue in perioperative monitoring. Among medical specialties, anesthesiology has been a pioneer in review and analysis of perioperative mishaps and implementation of improved monitoring techniques and guidelines. These monitors include pulse oximetry, which measures oxygen saturation in a pulsating vessel (SpO2), and capnography, which measures end-tidal carbon dioxide (ETCO2) level (Kossick, 2014). These advances have resulted in significant decreases in morbidity and mortality (Matten and Deshur, 2012).
The ASA (2011) amended its Standards for Basic Anesthetic Monitoring (Patient Safety) as guidelines for patient care. Perioperative nurses should be familiar with these standards and understand their significance in patient safety. If routine or frequent deviations from such standards occur, a performance assessment and improvement (also known as quality assurance [QA]) review along with a risk management analysis should be considered to ensure safe and high-quality patient care (ECRI Institute, 2009).
Monitors and basic anesthetic monitoring include the following:
• Inspired oxygen analyzer (FIO2), which is calibrated to room air on a daily basis
• Low-pressure disconnect alarm, which senses pressure in the expiratory limb of the patient circuit
• Respirometer (these first four devices are an integral part of most modern anesthesia machines)
• Blood pressure (usually measured with a noninvasive automated unit)
• Precordial or esophageal stethoscope
• Peripheral nerve stimulator if muscle relaxants are used
• SpO2
• ETCO2
Newer models of anesthesia machines have basic monitors integrated into a computerized system. These generally include FIO2; inspired and expired CO2; inspired and expired volatile agents; airway pressure and disconnect alarms; tidal volume, respiratory rate, and minute ventilation; noninvasive blood pressure (systolic, diastolic, and mean); SpO2 and pulse rate; temperature; and an event marker. A sophisticated, prioritized system displays caution or alarm conditions in one location, making it unnecessary to scan numerous individual monitors with a variety of displays when an alarm sounds. Perioperative nurses ensure that all appropriate monitor alarm systems are on and active, and that issues of “alarm fatigue” (Welch, 2012) have not induced the anesthesia provider to shut off these alarms.
Based on the cardiovascular and pulmonary status of the patient, scheduled surgical procedure, and the chance of significant physiologic changes, additional invasive monitors may prove necessary. These include direct arterial and venous pressure measurements, a pulmonary arterial catheter (PAC), and continuous mixed venous O2 saturation (SvO2) measured with a special PAC. One type of PAC provides a continuous measurement of cardiac output, using pulsed thermodilution to provide intermittent heat along a distal segment of the catheter. Small changes in the temperature of the blood are proportional to blood flow (cardiac output). These changes are sensed by a thermistor on the tip of the catheter (Contrera et al, 2014).
For certain conditions, equipment such as transcutaneous O2 and CO2 monitors, transesophageal echocardiography (TEE), evoked potentials, EEG, and cerebral or neurologic function monitors may be required. An indwelling urinary catheter is frequently inserted to provide a useful indication of renal function and hemodynamic status.
For procedures posing a risk of venous air embolism, a Doppler probe may be used. A central venous catheter is inserted, and the probe is placed over the right side of the heart, along the right sternal border between the third and sixth intercostal spaces. Positioning is confirmed if there is a change in Doppler signal after a 10-mL bolus of saline is rapidly injected through the catheter. The signal is monitored for the sound of a “mill-wheel” murmur. TEE is the most sensitive method of detection, but may also be the most expensive (Ranalli and Taylor, 2014).
Somatosensory evoked potential (SEP) monitoring may be used during some neurosurgery procedures (Chawla, 2012). It is used widely to assess the integrity of the spinal cord during surgery in which the spinal cord is manipulated. Upper and lower extremities may be monitored. Electrodes are usually placed preoperatively, but occasionally may be placed in the OR, before administration of anesthesia. Ischemia of ascending somatosensory pathways produces a drop in amplitude or loss of waveforms, thus warning the surgeon to take corrective action. Ischemic changes are usually widespread; rarely, however, is motor function lost when somatosensory pathways have not been affected.
Despite some controversy, most anesthesia providers believe that the monitoring used depends on the physiologic status and stability of the patient, the surgical procedure planned and its potential for sudden changes in cardiopulmonary functions, the anticipated blood loss and major fluid shifts, and the anticipated monitoring needs for postoperative management. Although not yet a standard of care, many facilities use the BIS monitor. This monitoring modality is a processed EEG obtained noninvasively from scalp electrodes. It provides a measure of the sedative and hypnotic effects of anesthetic medications on the central nervous system (CNS). Monitoring of some parameters may be negated by the anesthetic technique selected (Clark and Curdt, 2014).
Pulse Oximetry
Pulse oximetry works by analyzing the pulsatile arterial component of blood flow, thereby ensuring that arterial saturation (SpO2) rather than venous saturation is being measured. Two wavelengths of light are used, usually 660 nm (red) and 940 nm (infrared), because oxygenated and deoxygenated blood absorbs light quite differently at these wavelengths. At 660 nm, HbO2 (oxygenated hemoglobin) absorbs less light than HbR (reduced hemoglobin, or deoxyhemoglobin) does, whereas the opposite is observed with infrared light. Two diodes emitting light of each wavelength are placed on one side of the probe and a photo diode that senses the transmitted light on the opposite side. The amount of light absorbed at each wavelength by the pulsatile arterial component (AC) of blood flow differentiates itself from baseline absorbance of the nonpulsatile component and surrounding tissue (DC) (Miller, 2010). Given that absorption by other tissue components is essentially constant, the major variable is saturation of hemoglobin with O2. An internal microprocessor analyzes variations in absorption of light emitted from both light-emitting diodes (LEDs) and provides a readout of the percent saturation of hemoglobin with O2. Pulse rate also is given. The pulse oximeter converts the detected light to a plethysmographic signal that measures the drop in light intensity with each beat (Bozimowski, 2014).
The O2 dissociation curve indicates the percentage of totally saturated hemoglobin with O2. The following values are approximations (the O2 saturation [SpO2] values are percentages and the PaO2 levels are in torr): 98% to 100% (≥95 torr), 90% (60 torr), 75% (39 torr), 50% (26 torr), and 25% (16 torr). Most pulse oximeters are accurate to within ±2% greater than 70% and ±3% from 50% to 70% but correlate poorly at less than 50%. When breathing room air, the SpO2 for a young, healthy individual should be 98% to 100%; the SpO2 percentage for an elderly patient may be in the low 90s, whereas the percentage for a heavy smoker or a patient with severe lung disease may be in the 80s. It is wise to establish a baseline SpO2 value of a patient before any O2, medications, or stimulation is introduced. Maintenance of SpO2 levels greater than 90% corresponds to a PaO2 value of 60 torr or greater.
The pulse oximeter reading (often referred to as “pulse ox”) can be adversely affected by any event that significantly reduces vascular pulsations, such as hypoperfusion, hypotension, hypovolemia, vasoconstriction, or hypothermia. Electrosurgery, motion, or ambient light may also cause artifacts that will decrease the readout falsely. Carboxyhemoglobin (carbon monoxide bound to hemoglobin) falsely elevates indicated SpO2 saturation, whereas methemoglobin (hemoglobin that has an oxidized iron molecule and cannot reversibly combine with O2) falsely lowers SpO2 measurements. IV dyes may affect the pulse oximeter. Methylene blue may cause a drop to 65% for 1 to 2 minutes; indigo carmine, a very slight decrease; and indocyanine green, a slightly greater decrease. Nail polish also can decrease SpO2 values. Blue, black, or green polish significantly decreases the SpO2 value, whereas red polish has only a slight effect. Opaque, acrylic nail coverings may block the light beam. Some studies suggest the more advanced monitors have virtually eliminated these effects (Bozimowski, 2014). If nail polish or coverings seem to cause problems, the sensor can be turned sideways so that the fingernail is parallel to the light path.
The sensor usually is placed on a finger or a toe. Some manufacturers have sensors for the earlobe and the bridge of the nose, as well as smaller ones for soles and palms of infants and children. Modern devices are calibrated against laboratory SaO2 down to 70% saturation, and lower saturations are determined by extrapolation of the curve. Thus the user cannot calibrate pulse oximeters, and their reliability is dependent on the quality of signal processing and the stored calibration curve. Care must be taken to prevent localized neurovascular or ischemic damage. For example, a hard-cased sensor placed on a finger may cause ischemia when the arms are tightly secured at the patient’s side during a long procedure.
If trouble with the pulse oximeter arises with use of a local anesthetic, the perioperative nurse should evaluate the patient’s ventilatory status, verify proper placement of the sensor, and rule out the factors just listed that may adversely affect operation of the unit. Pulsatile blood flow in the extremity may be inadequate because of hypovolemia, decreased cardiac output, malpositioning, constriction by the blood pressure cuff, or hypothermia. As a final step the nurse can place the sensor on his or her own finger to verify satisfactory function of the pulse oximetry unit, cable, and sensor.
Capnography
A capnometer measures CO2 concentration; it produces a capnograph that displays the CO2 waveform. The capnograph provides a continuous display of the CO2 concentration of gases from the airways. CO2 concentration at the end of normal exhalation (ETCO2, PETCO2) is a reflection of gas from the distal alveoli; it therefore represents an estimate of alveolar concentration (PACO2). When ventilation and perfusion are well matched, the PACO2 closely approximates the PaCO2, and PACO2 ≅ PaCO2 ≅ PetCO2 (where PaCO2 is partial pressure arterial, PACO2 is alveolar partial pressure, and PETCO2 is end-tidal partial pressure). The normal gradient between PaCO2 and PETCO2 is more than 6 mm Hg. The gradient between PaCO2 and PETCO2 increases when pulmonary perfusion is reduced or ventilation is maldistributed (Hagberg, 2013). Under general anesthesia the gas is sampled at the point where the endotracheal tube (ETT) connects to the patient breathing circuit. During other types of anesthesia, in which oxygen is administered via a nasal cannula, a small-bore tube is connected to the cannula, which is then attached to the anesthesia machine so that ETCO2 levels can be measured. CO2 analyzers use various principles to measure CO2 in the inspired and exhaled gases on a breath-to-breath basis and display the CO2 waveform by mass spectrometry, infrared absorption spectrometry, or Raman scattering. Although capnography typically distinguishes tracheal from esophageal intubation, false-negative results (i.e., ETT in trachea, absent waveform) and false-positive results (i.e., ETT in esophagus or pharynx, present waveform) have been reported (Hagberg, 2013).
Compact units that provide a continuous indication of the ETCO2 level are the most widely used. These units measure the amount of infrared light absorbed by CO2 in the sample of gas. Two types of monitors are in use. In the mainstream unit, all respired gas passes through the detector, whereas in the sidestream unit, a portion of the gas is aspirated at a constant rate (50 to 250 mL/min) through small-bore tubing into the unit. Each design has advantages. Most units display a waveform of expiratory CO2 partial pressure relative to time after a short sampling and processing delay. The waveform is important to interpret output data correctly. Digital readouts usually give ETCO2 and respiratory rate. Daily user calibration is rarely required with newer units. The units confirm proper endotracheal intubation and are useful to detect anesthesia circuit disconnection, alveolar ventilation, early return of respiratory function after muscle relaxants are used, and acute alterations in metabolic functions, such as malignant hyperthermia (MH) or thyrotoxicosis (Bozimowski, 2014).
Anesthesia Machines and Anesthetic Gases
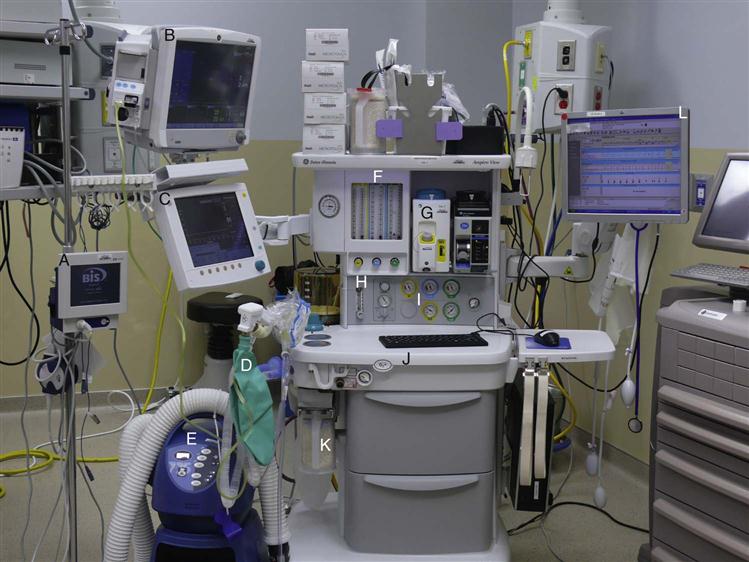
The first apparatus resembling an anesthesia machine was used in 1905. Since then innumerable changes and improvements have been made. Anesthesia machines look complicated, but the basic functions are similar and simple to understand. Perioperative nurses should be familiar with the basic function of anesthesia machines (Figure 5-1) because they may need to administer O2 during procedures with local anesthesia or conscious sedation/analgesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree