Chapter 13 Pleura and chest wall
Normal structure and function of the pleura
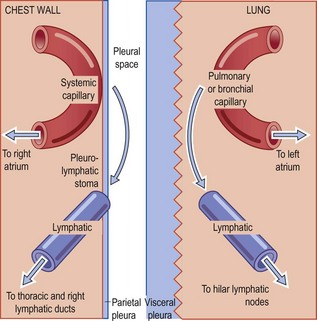
The lung is surrounded by a smooth glistening membrane, the visceral pleura, which is reflected at the hilum over the mediastinum and the inside of the chest wall as the parietal pleura. The potential space thus created extends from the root of the neck, 3 cm above the midpoint of the clavicle, down behind the abdominal cavity and the kidney as the costodiaphragmatic recess as far as the 12th rib. The two layers of the pleura are in close contact and slide easily over each other because of a thin mucinous film between them. Although this facilitates respiratory movements, there is little respiratory embarrassment if the pleural space is obliterated. Indeed, elephants and other large animals that generate large negative intrathoracic pressures lose their pleural cavities early in development without suffering any evident respiratory disability. The pleural cavities have therefore been called ‘an anatomical luxury and a pathological hazard’.1
Each pleural surface consists of connective tissue covered by a single layer of flattened mesothelial cells which secrete fluid rich in hyaluronic acid. However, most of the pleural fluid (which normally amounts to no more than a few millilitres) is a transudate of blood plasma with a protein concentration of less than 2 g/dl, mainly albumin. Although the parietal and visceral layers of the pleura both receive blood from systemic arteries – distal branches of the intercostal and bronchial arteries respectively – the visceral layer is largely supplied by pulmonary vessels and, because of the different pressures in the systemic and pulmonary circulations, most of the pleural fluid enters through the parietal pleura. Initial views that it was absorbed by the visceral pleura were based on the false assumption that the two pleurae were morphologically similar and that Starling principles would apply. In fact the parietal and visceral pleurae differ considerably in structure.2,3 Whereas the connective tissue of the parietal pleura is thin and capillaries and lymphatics within this tissue are no more than a few microns from the pleural space, the connective tissue of the visceral pleura is relatively thick. The pulmonary blood vessels lie deep to the pleura and are separated from the pleural space by as much as 60–70 µm. Furthermore, the parietal pleura is equipped with specialised stomata, described below, which provide a direct exit from the pleural space.4 Under normal circumstances most of the fluid therefore both enters and leaves the pleural space through the parietal pleura (Fig. 13.1).
The uptake of macromolecules and particles less than 4 nm in diameter probably takes place by passive diffusion through the gap junctions that connect many mesothelial cells5 and by active vesicular transport across the mesothelial cytoplasm.6,7 Larger particles are mainly cleared in the caudal portions of the parietal pleura. The systemic lymphatic network is particularly rich here, and mesothelial and lymphatic endothelial cells are in direct apposition, without an intervening basement membrane. Here too, stomata in the parietal pleura provide a direct connection between the pleural cavity and the underlying lymphatics.8 Over the lower mediastinum the stomata are associated with collections of macrophages and lymphoid cells, visible macroscopically as pale spots known as Kampmeier’s foci. Particles up to about 10 µm are absorbed by the stomata-membrana-cribriformis complex, a process that is monitored by the lymphoreticular cells of Kampmeier’s foci. Many particles are rapidly cleared by this route9 but some are retained in Kampmeier’s foci to form what are termed black spots.10–12
Mesothelial cells express epithelial markers such as cytokeratin and are joined by desmosomes which contribute to the formation of either tight or gap junctions. However, many mesothelial cells merely overlap one another, like lymphatic endothelial cells. Pinocytotic vesicles are plentiful in the cytoplasm which also contains rough endoplasmic reticulum and bundles of fine filaments. The mesothelial cell surface is not smooth but bears long, slender microvilli measuring up to 3 µm in length and 0.3 µm in width, suggesting an absorptive function. The numbers of microvilli vary from cell to cell. They are sparse over the ribs but increase towards the base of the lung and are more numerous on the visceral than the parietal pleura.13 With their surface mucopolysacharide coat, microvilli may also counter frictional forces.14
Except at the stomata in the parietal pleura described above, both layers of mesothelium rest upon a continuous basement membrane, beneath which there is a connective tissue network, which, as noted above, is thicker in the visceral pleura than in the parietal pleura. In the parietal pleura the amount and distribution of elastin are irregular whereas in the visceral pleura a thin inner and a thick outer elastic lamina can generally be discerned. Thus, the visceral pleura comprises five layers (Fig. 13.2):
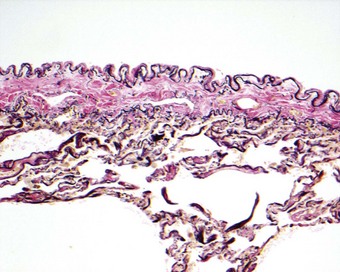
Figure 13.2 The normal visceral pleura at its intersection with an interlobular septum. The visceral pleura comprises five layers, the presence and thickness of which vary regionally: (1) the surface mesothelium and its basement membrane; (2) a thin layer of connective tissue; (3) a prominent outer elastin layer; (4) a band of collagen; and (5) an inner elastin layer (which is continuous with the alveolar elastin). Whereas the outer layer of elastin forms a continuous band around each lobe, the inner elastin layer turns inwards wherever interlobular septa join the pleura. In decortication it is the collagen layer that forms the plane of cleavage but it is the outer elastin layer that needs to be addressed when assessing tumour spread (see tumour staging, p. 571).
Mesothelial cells are readily damaged and desquamate in large numbers in many pathological conditions. Unlike epithelium, mesothelial repair does not solely involve centripetal migration of adjacent cells, as evidenced by the fact that large and small pleural lesions heal in exactly the same time. Mesothelial repair depends upon the differentiation of submesothelial connective tissue cells15–19 and exfoliated mesothelial cells resettling on the denuded areas20,21 as well as the centripetal migration of adjacent mesothelial cells.21,22 Macrophages also settle out from the pleural fluid23,24 but do not appear to differentiate into mesothelial cells.15,25 Differentiation of proliferating submesothelial cells involves the acquisition of cytokeratin antigens, markers of mesothelial cells not normally found in connective tissue; this complicates the interpretation of immunocytochemical stains used to distinguish granulation tissue from mesothelioma of sarcomatous pattern (see p. 728).17
Pleural effusions
Pleural effusions may be serous (hydrothorax), bloody (haemothorax) or chylous (chylothorax), or consist of frank pus (pyothorax or empyema thoracis). Most are caused by malignancy or infection.26 Their rapid therapeutic removal occasionally alters pulmonary haemodynamics to such an extent that pulmonary oedema or even pulmonary haemorrhage results.27,28
Hydrothorax
Serous effusions may be clear or cloudy, the former usually denoting a transudate low in protein content and the latter a protein-rich exudate. The traditional demarcation point between transudates and exudates is 3 g protein/dl but pleural fluid-to-serum ratios of protein, lactic dehydrogenase and cholesterol are now widely used.29–31
Exudates are of varying aetiology, being the result of pleural or pulmonary irritation from any cause. The irritation is often due to acute infection, which is usually pneumonic but may be a pulmonary or subdiaphragmatic abscess. Other causes include acute pancreatitis,32 rheumatoid disease, lupus erythematosus, tuberculosis, pulmonary infarction, a drug reaction (notably to practolol, methysergide and bromocriptine), non-malignant asbestos pleurisy, cancer and lymphatic obstruction or deficiency, and even pleural amyloidosis.33 Acute irritation typically leads to an accumulation of neutrophils in the fluid and chronic disease to increased numbers of lymphocytes. Pleural exudates due to defective lymphatic drainage are also rich in lymphocytes. A predominance of eosinophils is occasionally found, even in the absence of blood eosinophilia. The most common associations of an eosinophil-rich pleural effusion are malignancy, infection and trauma but often no cause can be identified.34 Exudates caused by cancer and pulmonary infarction are commonly blood-stained.26 The presence of tumour markers in the exudate may contribute to the diagnosis.35
Ascites may also result in pleural effusion due to ascitic fluid crossing the diaphragm, in which case the nature of the pleural effusion is similar to that of the ascitic fluid from which it derives. The passage is through mesothelium-lined channels, which are generally microscopic but may measure up to 5 mm.36 The sudden development of a pleural effusion in association with ascites secondary to a pelvic tumour is known as Meig’s syndrome (see p. 495). Hydrothorax is also recorded in patients with ascites secondary to cirrhosis36 and in those undergoing peritoneal dialysis.37 Ovarian stimulation undertaken for in vitro fertilisation is a more recently recognised cause of pleural effusion accompanying ascites.38
Chylothorax
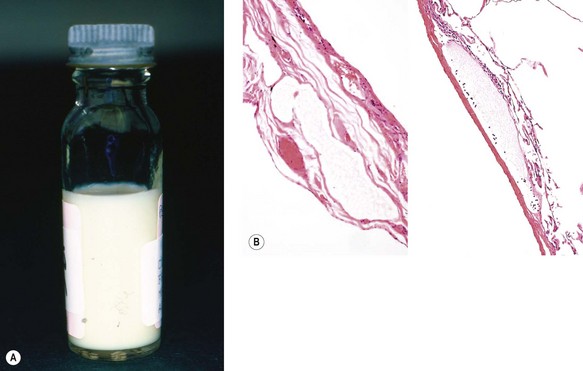
The accumulation of chyle in the pleural space is due to obstruction or rupture of the thoracic duct.39 Rupture is usually traumatic whilst obstruction may be developmental, neoplastic or surgical, or due to rare conditions such as lymphangioleiomyomatosis (see p. 293), angiofollicular lymphoid hyperplasia (Castleman’s disease, see p. 669) or the yellow-nail syndrome.40 Chyle is rich in dietary fat and a chylous effusion appears milky (Fig. 13.3A). Diagnosis involves cholesterol and triglyceride measurement in the pleural fluid. Pseudochylothorax may develop in a long-standing serous exudate: this also looks milky and has a high content of cholesterol but it lacks the triglycerides and chylomicrons found in chyle.41 Complications include malnutrition, immunosuppression and respiratory distress.
Yellow-nail syndrome
This syndrome, first described in 1964, consists of chylous effusions, lymphoedema of varied distribution and yellow discoloration and distortion of the nails, all of which are attributed to defective lymphatic drainage (Fig. 13.3B).42 It is often associated with repeated pulmonary infections, bronchiectasis and an immunological disorder such as lymphopenia, hypogammaglobulinaemia or macroglobulinaemia.42–47 Although widely regarded as representing a hereditary disorder, the family history is generally negative and the clinical features are seldom evident before adult life: the median age at diagnosis is 61 years (range 18–82 years).48,49 The yellow nails may return to normal if the pulmonary problems are treated successfully.49
Haemothorax
Haemorrhage into a pleural sac may follow trauma to the chest, pulmonary embolism or spontaneous rupture of an aneurysm of the thoracic aorta.50 Occasionally, rupture of an emphysematous bulla is followed by haemopneumothorax, with blood and air escaping simultaneously from the damaged lung into the sac.51 Thoracic endometriosis may also cause haemopneumothorax (see p. 495). Carcinomatous involvement of the pleura typically causes a blood-stained pleural effusion but seldom gives rise to frank haemorrhage.
Biliothorax (thoracobilia)
The removal of gallstones from the pleural cavity is one of the more unusual thoracic operations.52 Such calculi enter the thorax by producing a cholecystopleural fistula. The accompanying bile is liable to cause a chemical pleuritis resulting in severe respiratory insufficiency.
Pneumothorax
Clinical features
Pneumothorax is usually sudden in onset, and often occurs during physical exertion. It is generally unilateral. Bilateral pneumothorax is more serious since there is interference with the inflation of both lungs. The onset is painful, and quickly followed by shortness of breath, especially in patients with emphysema or other extensive chronic pulmonary disease that already impairs their respiratory capacity. In the cases in which the tissues create a valve at the site of perforation, the pneumothorax may build up slowly without any sudden symptoms; in these instances the displacement of the mediastinum is apt to be greater.
Epidemiology
Epidemiological studies show that men are affected more than women (in a ratio of 2.4:1), that there is a biphasic age distribution and that most deaths occur in older patients.54 Cigarette smoking increases the risk in a dose-dependent manner55 and cannabis use further increases this risk.56,57
Pathogenesis and pathological features
Escape of air into the pleural space from the lungs may be due to trauma or some pathological change in the lungs, usually emphysema. It causes the lung to collapse and if bilateral may prove fatal (see Fig. 7.2.7, p. 377). Other conditions in which pneumothorax occur particularly frequently are listed in Box 13.1. A sudden drop in atmospheric pressure may be the precipitating factor and occasionally loud music has also had this effect, pressure changes presumably being again responsible.58,59
Pneumothorax also occurs in healthy young individuals who appear to have normal lungs. This is known as idiopathic spontaneous pneumothorax. When treatment in such cases has necessitated thoracotomy it has usually been found that at the apex of the lung there is an area of fibrosis, 2–3 cm in its greatest dimension, surmounted by one or more bullae up to 1 cm or so in diameter.60,61 A hole may be found in the pleural surface adjoining the bullae, or one of the bullae may have a tear in its wall. Microscopy shows subpleural alveolar collapse and fibrosis, and an accumulation of chronic inflammatory cells (Fig. 13.4), but no evidence of tuberculosis or other specific disease. Such lesions are not uncommon. They were identified in 15 (6%) of 250 young, healthy adults undergoing bilateral thoracoscopic sympathectomy for hyperhidrosis.62 It has been suggested that, since most of these patients are tall, thin men, the changes in the apical region of the lungs – demonstrably affected bilaterally in some cases – may be developmental in origin and possibly related to rapid somatic growth. Their tall build will result in the apices of these individuals’ lungs being particularly poorly perfused when they stand erect. This ischaemia may damage the apices directly or contribute to the fibrosis by impairing the resolution of any inflammatory process.63 Because the apical changes are frequently bilateral, there is often recurrence on the opposite side.
Pneumothorax causes a reactive mesothelial hyperplasia associated with an inflammatory infiltrate that is frequently rich in eosinophils and is sometimes called `reactive eosinophilic pleuritis’.64,65 The infiltrate is superficial, penetrating only a short distance into the substance of the lung beneath the pleura: this distinguishes the process from eosinophilic granuloma of the lung and eosinophilic pneumonia. Small blood vessels may be involved but limitation of the process to the subpleural region of the lung distinguishes it from a true vasculitis. Reactive type II pneumocyte hyperplasia may also develop and be atypical enough to arouse suspicion of adenocarcinoma.66
If air is maintained in the pleural cavity for many months, as was once customary in the treatment of pulmonary tuberculosis, the mesothelium may undergo extensive squamous change. It is uncertain whether this represents metaplasia of the mesothelium, extension of metaplastic bronchial epithelium through a bronchopleural fistula or implantation of skin, but progression to malignancy has been noted.67 In cystic fibrosis, focal replacement of the visceral pleural mesothelium by ciliated columnar or squamous epithelium has been described, possibly due to rupture of bronchiectatic abscesses68 and perhaps contributing to the pathogenesis of pneumothorax in this condition.69 Unexplained columnar or mucous cell metaplasia of the pleura has also been reported.70
Pleurodesis
If a patient suffers from repeated pneumothoraces, it may be necessary to obliterate the pleural cavity, a procedure termed ‘pleurodesis’. This may be effected surgically, by stripping the pleura, or by the instillation of sclerosing substances such as talc or tetracycline. Intractable pleural effusions may also require such treatment, especially in the palliation of malignant effusions. However, before performing pleurodesis consideration should be given to the possibility of the underlying condition requiring lung transplantation at some future date as pleurodesis would make this technically difficult. The histological observation of numerous birefringent crystals in a fibrotic pleura indicates that pleurodesis has been undertaken in the past (Fig. 13.5). Neoplasia involving the chest wall 2 years after talc pleurodesis is recorded but the tumour was an adenocarcinoma of the peripheral lung rather than a pleural mesothelioma71 and is therefore likely to have been coincidental, particularly as no mesotheliomas developed in any of 80 patients who had undergone talc pleurodesis 22–35 years previously.72 Although commercial talc is often contaminated by tremolite asbestos and talc miners have developed mesothelioma, cosmetic talc and that used in medicine are rigorously screened to ensure their purity; they appear to be entirely non-carcinogenic but there are reports of acute lung enjury following pleural talc insufflation.73,73a
Inflammation and fibrosis of the pleura (pleuritis, pleurisy)
Inflammation of the pleural sac can develop in many conditions (Box 13.2). Usually, it is secondary to inflammation of one of the adjoining thoracic or subdiaphragmatic structures, particularly the lungs. Sometimes, it is a manifestation of systemic lupus erythematosus or another connective tissue disorder (see below). Asbestos and certain drugs may also cause pleural inflammation and fibrosis (see pp. 715 and 391). Silicotic nodules developing along the pleural lymphatics have been likened to pearls or drops of candle wax74 while eosinophilic pleuritis as a reaction to air in the pleural cavity has been mentioned above. Occasionally, no cause is evident.75
In many cases of serofibrinous pleurisy the inflammation regresses as time or treatment brings about recovery from the underlying disease. In such cases, the exudate is absorbed, and unless the pleura has been extensively denuded of its mesothelium the sac recovers its smooth lining. Where mesothelium has been lost, adhesions may form between the lung and the chest wall: at first the adhesions are fibrinous, but later, through organisation, they become fibrous. In this way, part or all of the cavity may be obliterated. This generally occasions no great functional disadvantage. However, an appreciable degree of diffuse fibrous thickening of the pleura of any cause impairs movement of the chest. Accordingly, bilateral diffuse pleural fibrosis has important clinical consequences. The causes are as numerous as those of pleuritis (see Box 13.2) but asbestos is particularly important and the condition is therefore dealt with below under the heading of non-malignant asbestos-induced pleural disease.
The organisation of surface fibrin often involves the development of abundant granulation tissue and this may mimic sarcomatoid mesothelioma. The expression of keratin by such spindle cells is generally taken as evidence that they are mesothelial rather than fibroblastic, but it is not necessarily indicative of mesothelioma because reactive submesothelial spindle cells stain similarly.17 However, cytokeratin staining is a useful marker of malignancy if it is positive in irregular cells in the deeper parts of the process for, as granulation tissue matures away from the surface, the intensity of the cytokeratin staining lessens.
Empyema thoracis (pyothorax)
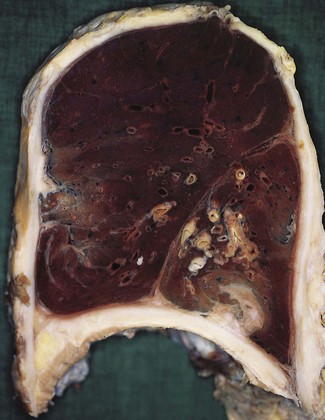
Empyema thoracis represents a collection of pus in the pleural space (Fig. 13.6). It is usually a complication of pneumonia and, with the advent of antibiotics, is now rare. When it does occur there is usually some underlying debilitating condition such as diabetes or alcoholism, chronic lung disease such as bronchiectasis or carcinoma, or the patient is on long-term immunosuppressive therapy. In about a third of cases the condition is associated with a necrotising pneumonia or ‘primary’ lung abscess, due to aspirated anaerobes (see pp. 189–191). Often there is a mixture of bacteria, the predominant ones being the anaerobic or microaerophilic Streptococcus milleri, peptostreptococci, peptococci, Fusobacterium nucleatum, Bacteroides melaninogenicus, B. fragilis, clostridia, Escherichia coli and Pseudomonas aeruginosa.76–79 Klebsiella pneumoniae is common in patients with diabetes mellitus80 whereas in children Staphylococcus aureus is common, the empyema generally complicating underlying staphylococcal pneumonia or pneumatocele (see p. 182). If it complicates a staphylococcal pneumatocele the empyema may be associated with pneumothorax. This combination is known as pyopneumothorax and may also develop when a cavitating rheumatoid nodule establishes a bronchopleural fistula.81,82 Empyema may also follow thoracotomy, or less commonly thoracocentesis, penetrating injuries or rupture of the oesophagus during instrumentation or by a sharp foreign body. Blood-borne and transdiaphragmatic infection are rare causes of empyema.
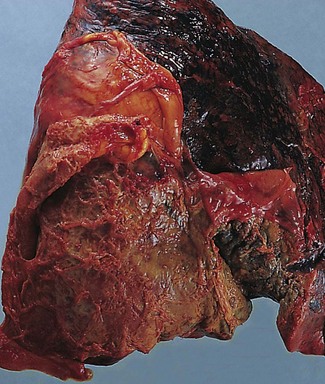
Figure 13.6 Empyema thoracis. There is shaggy fibrinopurulent thickening of both pleural surfaces over the lower lobe.
In empyema thoracis the whole of the sac or merely a part, usually the lower part, may be filled with thick viscous pus (see Fig. 13.6). Sometimes the pus is confined to an interlobar fissure. Organisation of parts of the exudate leads to the formation of firm adhesions that restrict spread of the infection and localise the pus. Alternatively, the pus may extend into the lung and rupture into a bronchus, leading to the sudden dissemination of infected material throughout the bronchial tree. If the patient survives this, there remains a chronic bronchopleural fistula. Occasionally, the pleural infection erodes the chest wall, leading to discharge of pus through the skin (empyema necessitans).
Empyema thoracis is a serious condition which, if inadequately treated, has a high mortality. Sometimes infection is overcome without intervention, and the pus is eventually replaced by granulation tissue and fibrous tissue. Even in favourable cases the damage to the serosa is severe: when the suppuration subsides, fibrous adhesions develop and eventually obliterate the cavity. The lining of the cavity may undergo calcification during the succeeding years. Malignant change is a further complication (see pleural sarcoma and pleural lymphoma, p. 734).
Tuberculosis of the pleura
In the early stages of a tuberculous pleurisy the appearance of the visceral and parietal serosal surfaces does not differ greatly from that seen in the usual serofibrinous form of pleurisy. Discrete miliary granulomas may stud the pleura but more often there is diffuse surface involvement. However, typical tubercles can be seen histologically. Pleural biopsy with both histological and bacteriological examination has a sensitivity of greater than 90%,83 confirming that the condition is infective rather than allergic. Ultimately, there is caseation and, in time, this may undergo calcification.
A more severe form of tuberculous infection of the pleura may develop in the course of postprimary tuberculosis if a tuberculous cavity erodes into the pleural sac. The result is the condition conventionally described as a tuberculous empyema (see Fig. 5.3.8, p. 202), although the semifluid matter that collects is caseous and not true pus, except when there is a simultaneous or subsequent infection by pyogenic bacteria. The exudate in the pleural sac becomes encased in a thick, fibrosing layer of tuberculous granulation tissue. The same condition may result from rupture of a juxtavertebral abscess in cases of tuberculosis of the thoracic spine. Similarly, tuberculosis of a rib may be complicated by pleural effusion or ‘empyema’. Bronchopleural fistula may be present in association with tuberculous ‘empyema’. This increases the liability to secondary infection, which in some cases is caused by fungi.84
The formerly common establishment of an artificial pneumothorax to rest a tuberculous lung has been mentioned above. Other manipulations favoured in the past for the same purpose included the resection of several ribs (thoracoplasty) or the introduction of some inert material into the pleural sac, a procedure known as plombage: decades later, patients may find worm-like threads of wax in their sputum,85 or pathologists unfamiliar with this outdated procedure may be surprised to find at autopsy one side of a deceased elderly patient’s chest largely filled with what look remarkably like white billiard balls. More often, complications such as infection led to the removal of the plombe within a few years of its insertion.86 Various forms of plombage material were favoured: plastic in the form of sponge or lucite spheres, packets of shredded plastic and injections of paraffin wax or vegetable oil (oleothorax).87 The development of carcinoma of the lung underlying lucite spheres is mentioned on page 735.
Connective tissue disorders
Pleural involvement is common in rheumatoid disease and systemic lupus erythematosus, but rare in systemic sclerosis, polymyositis and dermatomyositis.88
Rheumatoid disease
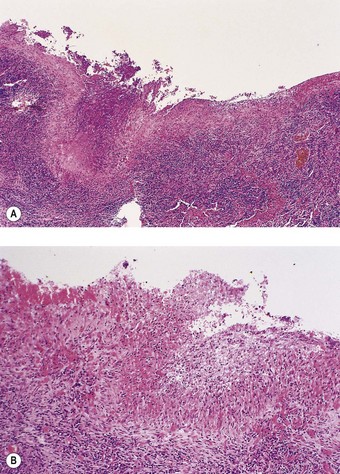
Pleurisy and pleural effusions are the most common of the several respiratory manifestations of rheumatoid disease (see Box 10.1, p. 473). The pleural inflammation may be non-specific or represented by classic rheumatoid nodules.89 Sometimes a subpleural rheumatoid nodule discharges into the pleural space and the characteristic palisade of cells forms a linear pattern over the surface of the pleura (Fig. 13.7).90 An effusion, unilateral or bilateral, generally forms. This is an exudate, rich in immunoglobulins and other proteins, and sometimes cloudy or opalescent due to a high cholesterol content. Glucose levels in the fluid are low, apparently due to impaired glucose transport.91 Lymphocytes are the predominant leukocytes, but sometimes there is a unique microscopical pattern – a combination of large elongated multinucleate cells, cholesterol crystals, clumps of eosinophilic granular material and a dearth of mesothelial cells.92–94 The elongated cells are believed to come from the palisade layer of the granuloma. Their bizarre shape makes them liable to be mistaken for carcinoma cells. Rheumatoid pleural effusions are liable to become infected, sometimes resulting in empyema. At other times a cavitating intrapulmonary rheumatoid nodule leads to the development of a bronchopleural fistula and pyopneumothorax.81,82
Nodular histiocytic/mesothelial hyperplasia
Nodular histiocytic/mesothelial hyperplasia is found on serosal surfaces as small discrete aggregates of histiocytes intermingled with occasional mesothelial cells. The condition is well known on the pericardium where the aggregates are often termed mesothelial/monocytic incidental cardiac excresences (cardiac MICE), and upon the peritoneum in hernial sacs. The aggregates are also found on the pleura, probably developing in response to non-specific pleural irritation.95 They are symptomless and of no clinical importance unless an unwary pathologist mistakes them for something more sinister, such as carcinoma or mesothelioma. Recognising the numerous histiocytes as such is the key to the correct diagnosis; these cells stain strongly for CD68 and greatly outnumber the calretinin- and cytokeratin5/6-positive mesothelial cells and any reactive thyroid transforming factor-1 (TTF-1)-positive pulmonary epithelial cells present at the deeper aspect of a lesion on the visceral pleura.96
Non-malignant asbestos-induced pleural disease
Besides asbestosis, carcinoma of the lung and pleural mesothelioma, respiratory disease attributable to the inhalation of asbestos includes pleurisy, which is generally accompanied by an effusion, and pleural fibrosis.97 The latter takes several forms: distinctive plaques and less distinct pleural fibrosis that may be focal or diffuse, and either of no clinical consequence or responsible for particular clinical syndromes.
In contrast to asbestosis and asbestos-induced lung cancer, both of which generally require relatively high exposure levels, all pleural diseases attributable to asbestos are associated with relatively low asbestos burdens in the lung.98 Furthermore, it is very difficult to identify asbestos in the pleural lesions themselves, although they are attributable to asbestos inhalation.
Benign asbestos pleurisy and pleural effusion
An exudative pleurisy may develop within 10 years of first contact with asbestos and therefore be found in persons currently at work in the industry. The effusion may be asymptomatic97 or the patient may complain of pleuritic pain, sometimes accompanied by fever, leukocytosis and an elevated blood sedimentation rate.99 The effusion is usually small and blood-stained, giving rise to suspicion of malignancy. It often resolves spontaneously, only to recur, perhaps on the other side. It may progress to diffuse pleural fibrosis that requires decortication.99 Asbestos-induced pleurisy is thought to play no part in the development of pleural mesothelioma.
Asbestos-induced pleural fibrosis
This thin fibrous thickening of the visceral pleura is of no clinical consequence but occasionally asbestos-exposed individuals develop extensive, thick fibrosis of the pleura in the absence of asbestosis (Fig. 13.8) and this may severely compromise expansion of the chest.24,100–104 It may be bilateral or limited to one side.105 The resultant restrictive defect in lung function is responsible for incapacitating breathlessness and this form of pleural fibrosis is one of the industrial diseases recognised by several governments for compensation purposes. Medicolegal definitions vary from country to country and with time. In the UK the current definition is unilateral or bilateral diffuse pleural thickening with obliteration of the costophrenic angle.106 Mesothelioma may develop in patients with such restrictive pleural fibrosis but the tumour is thought to arise independently. Although generally limited to the pleura, the fibrosis occasionally extends into the chest wall107 and mediastinum.108 The lung parenchyma immediately beneath the thickened pleura may show diffuse interstitial fibrosis but this should not be interpreted as asbestosis unless there is also evidence of substantial asbestos exposure.
Asbestos-induced pleural fibrosis has to be distinguished from pleural plaque, which is described below, and from a cryptogenic form of bilateral fibrosing pleuritis that has been reported in a few patients in whom asbestos, systemic connective tissue disorders, tuberculosis and all other recognised causes of pleural fibrosis have been excluded.109 Such cryptogenic diffuse pleural fibrosis has been reported in three sisters.110 In other patients it has been associated with a thin rim of subpleural fibrosis and been termed idiopathic pleuroparenchymal fibroelastosis.111,112
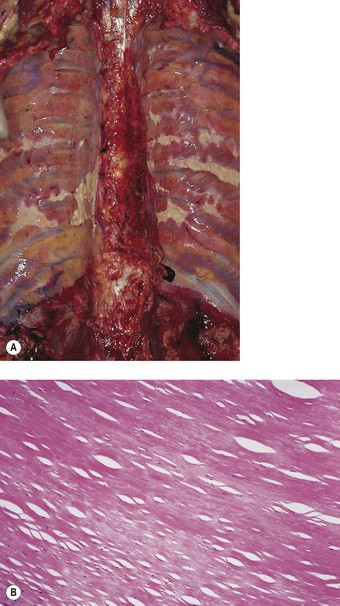
Blesovsky’s disease (folded lung, rounded atelectasis)
Localised patches of pleural fibrosis may be important clinically, not for their functional effects, but because they may mimic a peripheral lung tumour radiographically. The contracture of the pleural scar draws the adjacent lung tissue to it, causing a localised area of collapse. The bases of the lungs are particularly affected (Figs 13.8 and 13.9 and Table 13.1). The collapsed lung often appears to be folded upon itself and the resultant radiographic opacity has a comet’s tail appearance. The condition is variously known as Blesovsky’s disease, folded lung or rounded atelectasis.24,113–117a In 25% of cases the lesion is bilateral.105 There is often a history of asbestos exposure, but like the other forms of asbestos-induced pleural fibrosis, the condition is thought to play no part in the pathogenesis of mesothelioma.
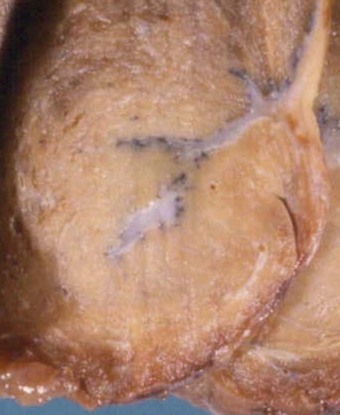
Figure 13.9 Folded lung (Blesovsky’s disease). A white Y-shaped band of scarred pleura is surrounded by collapsed lung.
(Courtesy of Dr T Jelihovsky, Sydney, Australia.)
Table 13.1 Lobar distribution of Blesovsky’s disease in 100 reported cases113
| Right | Left | |
|---|---|---|
| Upper lobe | 2 | 3 |
| Middle lobe/lingual | 11 | 10 |
| Lower lobe | 47 | 27 |
Pleural plaques
Pleural plaques are a further form of asbestos-induced pleural fibrosis, described separately because of their distinctive pathological appearance.24 Although they are occasionally found in persons with no history of asbestos exposure, the association with asbestos is a firm one, based on both epidemiological evidence118,119 and the identification of asbestos in the underlying lung.120 Pleural plaques therefore provide strong evidence of asbestos exposure, which may be occupational or environmental. They occur after exposure to all types of asbestos but anthophyllite is particularly potent. Plaques are common in areas where the soil is contaminated with asbestiform minerals, for example parts of Finland and Greece.121,122 The longer and heavier the exposure, the more extensive the plaques, but even slight exposure can be sufficient. Very few develop within 15 years of first exposure and most appear after 30 years. Postmortem studies show that plaques are more common than can be appreciated from radiological examination in life.118 Pleural plaques are harmless and have no relationship to mesothelioma, or lung cancer, other than their association with asbestos.123
Pathology
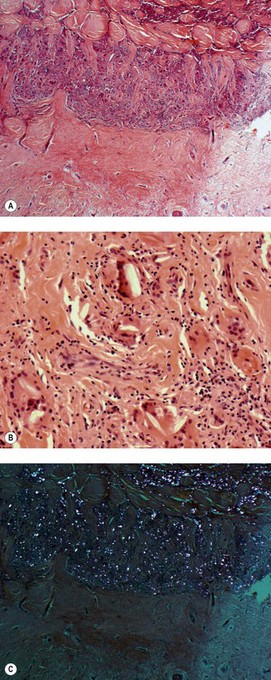
Plaques are localised raised areas that are generally multifocal and bilateral. They may occur on the visceral pleura or even the peritoneum124 but usually affect the parietal pleura, particularly in relation to the ribs and the central tendon of the diaphragm. They have an irregular outline, a smooth shiny pearly-grey surface and a low profile, typically about 5 mm in height: their other dimensions are very variable (Fig. 13.10A). Microscopically, plaques are hyaline, avascular and almost acellular (Fig. 13.10B). In a pleural biopsy, even a small number of spindle cells in a hyaline lesion should suggest the possibility of paucicellular hyaline mesothelioma (see p. 722) rather than plaque. The hyaline collagen bundles have a characteristic pattern that is often likened to basket weave. Calcification is common. Inflammation is not found, except at the periphery and there it is mild.
Pathogenesis
Although the connection between asbestos and plaques is firmly established, the pathogenetic mechanism is obscure. Organisation of exudates probably underlies the other less distinctive forms of pleural fibrosis found in asbestos workers but it is difficult to envisage a special type of exudate that may give rise to plaques. Furthermore, the plaques are covered by an intact mesothelium and are not usually attended by adhesions. It has been suggested that the parietal pleura is particularly affected because it is abraded by asbestos fibres projecting through the visceral pleura, but no such projections have been identified. A further possibility derives from consideration of the lymphatic drainage of the pleural cavity, which is largely through the parietal pleura. Short asbestos fibres have been identified in the visceral pleura125 and it is likely that some reach the pleural cavity, whence their disposal would be by the stomata and lymphatics of the parietal pleura (see p. 708). Any fibres arrested by the ribs or the tendinous portion of the diaphragm would elicit submesothelial fibrosis at these sites.24 Ferruginous bodies are not usually seen in the plaques but they have been recorded there.126
Pleural tumours
Pleuropulmonary blastoma is dealt with on page 623 and this section will consider only those tumours that are initially confined to the pleura, of which mesothelioma is by far the most important.
Mesothelioma
Mesotheliomas arise most commonly in the pleura but also develop in the peritoneum and occasionally in the pericardium or tunica vaginalis of the testis.127,128 They are malignant neoplasms: the terms ‘benign fibrous mesothelioma’ and ‘multicystic mesothelioma’ are misnomers in that the former is not of mesothelial origin and the latter is not neoplastic (see solitary fibrous tumour on page 730 and multicystic mesothelioma on page 729). A benign mesothelial neoplasm is encountered in the peritoneum and rarely in the pleura but is termed adenomatoid tumour rather than benign mesothelioma (see p. 729). Well-differentiated papillary mesothelioma is a low-grade mesothelioma that warrants separate consideration (see p. 729).
Incidence
Mesothelioma was very rare in the nineteenth and early twentieth centuries. Indeed, as late as the 1950s, some eminent authorities denied its existence. However, it is now recognised to be one of the most important occupational diseases. Its incidence has been steadily rising and in industrialised countries mesothelioma now accounts for about 1% of all cancer deaths. The highest annual crude incidence rates (about 30 cases per million) are observed in Australia, Belgium and the UK.129,130
Measures to curtail exposure to its principal cause, asbestos, were introduced and progressively strengthened in many countries in the latter half of the twentieth century but such is the long latent interval between exposure and disease that these do not have an effect for several decades. The durability of asbestos is another factor hampering efforts to reduce the incidence of mesothelioma. It is calculated that the numbers mesotheliomas in the UK will triple between 1991 and 2020 and only then decline.131 In contrast, there is already a decline in the incidence of mesothelioma in the USA where the use of amphibole asbestos was restricted as early as the 1960s.132
Aetiology
In the mid twentieth century it was increasingly suspected that mesothelioma was related to asbestos exposure and substance was given to this in 1960, when Wagner and colleagues showed a high incidence of the tumour in the area near Kimberley, northern Cape province, South Africa, where blue asbestos is mined.133,134 The relationship between asbestos exposure and mesothelioma was subsequently confirmed in other countries where asbestos is mined and in those that import and process the ore.135–139 Asbestos and similar fibrous substances are virtually the only recognised causes of mesothelioma. Smoking does not increase the risk of mesothelioma. Cases that can be attributed to causes such as radiation, chronic inflammation and scarring are exceptionally rare.140–146 Despite the predominant role of asbestosis, there is evidence that simian virus 40 (SV40) may also play a role in the development of mesothelioma, acting either independently or synergistically with asbestos. This virus contaminated monkey kidney cells used in the manufacture of early batches of poliomyelitis vaccine and may have reached humans in this way. SV40 sequences are selectively expressed in many mesotheliomas, the development of which has been attributed to the ability of the sequences to inactivate the p53 and Rb tumour suppressor genes and promote the secretion of various growth factors.147–150 However, subsequent investigations have resulted in both supportive and contrary findings151–156 and the relationship of SV40 infection to development of mesotheliomas remains uncertain.
The risk of mesothelioma is substantial in the mining, transportation and processing of asbestos but is dose-related and can be minimised by precautionary measures based on good industrial hygiene.157 The principal manufacturing industries using asbestos are those producing items such as specialised cement products, insulating materials and brake linings. All industries applying and refitting asbestos insulation carry a potential risk and many cases have been seen in construction and demolition work, ship-fitting and other dockyard work, and railway carriage maintenance. Many joiners, heating engineers, boilermen, railwaymen and former naval servicemen have been exposed unknowingly158 whilst some exposure is para-occupational, incurred by men such as shipyard welders who work alongside those applying or stripping asbestos. The families of asbestos workers are also at increased risk through being exposed to asbestos dust carried home on work clothes. Such exposure may occur very early in life.159 Others appear to have developed mesothelioma because they live in the vicinity of asbestos mines or factories, but it is always difficult to exclude occupational, para-occupational or domestic exposure in such cases.160,161 However, environmental exposure undoubtedly occurs in parts of the world such as Turkey and the Metsovo region of Greece where the soil contains asbestos,121,122,162,163 and town dwellers are subjected to continuous low levels of asbestos exposure from sources such as brake linings. Thus, exposure to asbestos may be:
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree