Fig. 9.1
Basic principles of U-FISH assay. The assay contains directly fluorescent dye-labelled single-strand DNA probes. Pre-analytical procedures include slide preparation (including pretreatment of pre-stained slides). UroVysion assay starts with probe preparation and specimen—target—DNA denaturation followed by DNA hybridization of probes to target DNA sequences. Analysis of FISH signals is performed under epifluorescent microscope. Legend: CEP centromere enumeration probe, LSI locus-specific identifier probe, chr. chromosome, loc. locus
Analysis of FISH Signals
An epi-fluorescence microscope equipped with a 100-watt mercury lamp and appropriate filters is recommended to detect multicolor fluorescent signals. Specimens should be scanned at 400× magnification to locate cells with nuclear signals and then analyzed under 600–1000× magnification. Systematic analysis of signals follows and a standardized and repeatable approach is essential (e.g., starting analysis in the upper left quadrant of the target area and continue scanning from left to right and top to bottom). Signals should be counted in abnormal cells, which are defined by their nuclear features, namely enlarged nuclear size, irregular nuclear borders, and “patchy” 4,6-diamidino, 2-phenylindole dihydrochloride (DAPI) staining. Besides the single cells, cell clusters may be evaluated avoiding counting signals in overlapping nuclei. The number of signals for all four probes should be counted for each abnormal cell and recorded only when there is a gain (≥2 signals) for two or more chromosomes 3 (red), 7 (green), 17 (aqua), or there is a loss of both copies of 9p21 (gold) as recommended by the manufacturer. Total number of abnormal cells analyzed should be recorded. The test is considered positive when ≥4 of the 25 analyzed cells show gains for two or more chromosomes or ≥12 of the 25 cells have zero 9p21 signals. If these criteria for test positivity are not met after viewing 25 cells, analysis should be continued until the entire sample is analyzed. Some authors have recommended optimization of the criteria with regard to the presence of benign tetraploid cells [2, 3]. Tetraploid, or less commonly octaploid, cells showing four or eight signals for each of the four FISH probes can occur in reactive cells (e.g., umbrella cells) and may give rise to false-positive FISH results. Thus, FISH results should not be considered positive based on a tetraploid pattern unless they are numerous (e.g., ≥10). Along this line, the FDA-approved package insert emphasizes that results at or near the cut-off point should be interpreted with caution. A schematic illustration of typical U-FISH findings is shown in Fig. 9.2.
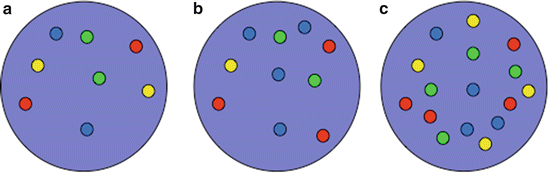

Fig. 9.2
Schematic illustration of U-FISH findings. (a) Nuclei of a FISH-negative benign cell with two signals for the chromosomes 3 (red), 7 (green), and 17 (aqua), and for 9p21 (gold). (b) U-FISH-positive cell with increased and unbalanced number of chromosomes 3 (red) and 17 (aqua) and heterozygous loss of 9p21 (gold). (c) Tetraploid FISH pattern with four signals of each FISH probe. This pattern is not specific for urothelial carcinoma but commonly seen in reactive urothelial cells
There are several conditions that may prevent accurate FISH examination, including the presence of lubricant jelly, corpora amylacea, degenerated cells, crystals, sperm, and bacteria.
In the case of performing FISH on conduit urines, interference by inflammation and cell debris may occur. Squamous contamination, especially in urines from female subjects, may obscure urothelial cells. If there are only a few atypical cells present, then a targeted FISH approach, using the same Papanicolaou-stained preparation as the cytology slide, is advisable.
Imaging and Automation of UroVysion® FISH Analysis
Advantages of an Automated Imaging System
Because manual FISH analysis has a relatively slow throughput and the fluorescent signals fade over time [4], automated imaging systems are increasingly being utilized, especially in institutions with high volumes of FISH tests. Reviewing representative digitized images instead of manual review of a FISH slide at a microscope can lead to dramatic savings in pathologist time [5]. Imaging systems may also be implemented for improved productivity, quality control, archiving of images, and increased accuracy. In a recent (2013) College of American Pathologists (CAP) proficiency test survey involving 192 laboratories, use of automated systems was estimated to be almost 50 %, with manual counting making up the remainder. In some labs there was a combination of both manual and automated use. In non-US countries, automated imaging systems for U-FISH analysis are less commonly used.
A high concordance (>98 %) between the manual method and the automated method of reviewing cells has been shown. In several cases deemed negative by the manual method, machine-assisted interpretation showed a positive result for abnormal cells which was subsequently verified within a short interval by cystoscopy [5]. In addition, the ability to enlarge cells with questionable signals and enhance weak signals is advantageous in reducing false-positive or false-negative results.
Disadvantages of automation may include a higher number of unsatisfactory specimens due to scant cellularity or clumping, in which case the manual system of review is employed. Factors to consider before purchasing an imaging system include financial cost, space requirements, information technology system needs or modifications, validation of system, training programs for personnel, continuing education, maintenance of system, workflow, and patterns of reimbursement [5].
Details of Automated Imaging Systems
Imaging systems consist of an automated scanning microscope coupled with software for image analysis. Some are approved or validated by the FDA for the detection, classification, and enumeration of urine specimen cells from individuals with a history of UC probed by the U-FISH Kit. Imaging Systems that have been approved by the FDA (as of 2014). These include the Duet TM System™ (Bio View, LTD; Figs. 9.3 and 9.4) and the Ikoniscope oncoFISH Bladder Test System (Ikonsys, Inc., New Haven, Connecticut). Technical details of these imaging systems are described in the Appendix. Automated systems allow for interactive rejection or acceptance of cells, so that overlapping cells or degenerated cells can be ignored [6]. The imaged cells are then formatted in a gallery on a screen for review. Normal and abnormal cells can be segregated and then reclassified as needed [5]. Extraneous cells such as neutrophils and lymphocytes are automatically excluded from analysis.
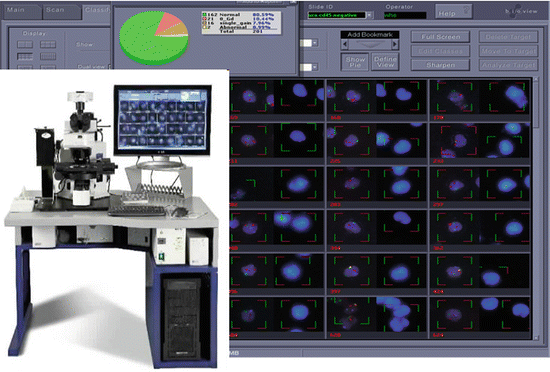
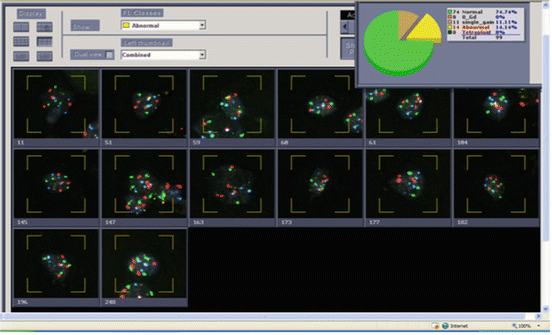

Fig. 9.3
Automated scanning instrument showing workstation with concurrent acquisition of DAPI images and FISH images illustrated on screen

Fig. 9.4
Urine from a patient with a history of high grade urothelial carcinoma. Histogram of Urovysion FISH abnormal cells showing 14 out of 25 cells with polysomy for at least two chromosomes per cell. This is an abnormal result and consistent with high grade urothelial carcinoma. Although a total of 99 cells were scored, reporting is based on 25 of the most morphologically abnormal cells scored
Target FISH
FISH may also be performed on Papanicolaou-stained cytospins that have been pre-scanned by the imaging system with atypical urothelial cells selected by the operator. The slide is subsequently destained with acid alcohol and hybridized with the four-color probe set. The hybridized cells are then matched in perfect relocation with the pre-selected cells so that these same cells can be analyzed for abnormal FISH signals. This is also known as target FISH, and was shown to be more accurate when compared to conventional cytology [7]. In laboratories that do not have an automated imaging system in place, targeted U-FISH analysis of atypical urothelial cells can also be achieved by usage of a standard fluorescence microscope equipped with an automated stage and a camera allowing for interactive automated relocation and imaging of representative cells for review and documentation [8]. Target FISH enables the reviewer to associate aneuploidy with cytologic features on a per cell basis.
Reporting of Results
Following examination of all cells a report summarizing the sample’s data is generated.
One or several pathologists can sign-out cases by using remote computers situated away from the main automated scanning microscope equipped with appropriate software [5, 9]. In manual screening a minimum of 25 abnormal cells are evaluated. In contrast, the automated systems require a minimum of 100 urothelial cells without any chromosomal aberrations to be classified as normal [5]. Using either method, only 25 of the most abnormal cells are reported.
Factors affecting the scanning performance include the cellularity, hybridization efficiency, and cleanliness of the slide and coverslip. In addition, slides with marked bacterial contamination [4] or obscuring inflammation are difficult to analyze. The CAP requires photographic or digitized images for all FISH assays which should include at least one cell image for assays with normal results and two cells images for assays with abnormal results (images of at least two cells are required to document all abnormalities since the multiple chromosome loci are evaluated in a single test). Images on all FISH assays testing for neoplastic conditions must be retained for documentation for a minimum of 10 years (CAP requirement CyG.43300). In addition, CAP requires that pathologists and technologists participate in proficiency testing [5].
Performance of UroVysion® FISH Testing
U-FISH testing of voided urine has been approved by the FDA as an aid for initial diagnosis of bladder cancer in patients with hematuria and/or subsequent monitoring for tumor recurrence in patients previously diagnosed with bladder cancer. In routine practice, there are several additional situations in which U-FISH is frequently used [8]. In a meta-analysis, the pooled sensitivity and specificity of FISH were 72 % and 83 %, respectively, as compared with 42 % and 96 %, respectively for cytology [10]. Putting published data of U-FISH results into the right perspective is challenging due to great variability of critical parameters; these include the type of specimen (voided urine versus bladder washing), clinical situation (history or no history of bladder cancer), concurrent cystoscopy findings, length and type of follow-up, proportion of low grade and high grade tumors, local experience in FISH analysis, and the definition of a positive result, among others. This emphasizes the need for a well-controlled and independently funded study to clarify the performance of FISH in clearly defined situations.
UroVysion FISH in Atypical Urinary Cytology
Atypical urinary cytology (AUC) has emerged as the most rewarding application of U-FISH analysis [2, 11–17]. In particular U-FISH is currently regarded as the most useful marker in the setting of a negative cystoscopy and atypical cytology according to the recent recommendations of the International Consultations on Urological Diseases and the European Society of Urology [1]. In a pivotal study, U-FISH in 120 urine specimens revealed a sensitivity of 100, 89, and 60 % in patients with suspicious, atypical, and negative cytology, respectively, while the overall specificity was 97 % [18]. This is in line with another study showing that the sensitivity of FISH to detect UC in patients with atypical cytology and equivocal or negative cystoscopy was 100 % with a specificity ranging from 60 to 100 % depending on whether or not there was a history of UC or a lesion at cystoscopy [15]. The low positive predictive value in patients with a history of UC and a negative cystoscopy in this study were explained by early detection of neoplastic cells that preceded the appearance of established cancer. In fact, in other studies, such “anticipatory positive” FISH results predicted recurrence in patients under surveillance with atypical or suspicious cytology and a negative cystoscopy [16, 17, 19]. Despite these promising data, performing FISH after an AUC result and a negative surveillance cystoscopy is not yet generally recommended since these results are unlikely to change the currently established surveillance strategies in non-muscle-invasive UC.
U-FISH is particularly helpful in the notoriously difficult field of atypical cytology after intravesical BCG treatment of high grade non-muscle-invasive UC [12, 20–22]. Patients with a positive post-BCG cytology or FISH result have a substantially higher risk of recurrence when compared to patients with negative results [12, 23]. Washing cytologies of the UUT can also be challenging due to instrumentation-related changes that might lead to false-positive cytology results. On the other hand, accurate diagnosis of UUT UC is critical to avoid delay of diagnosis. Published reports suggest that U-FISH also appears as an interesting tool to increase the sensitivity for the detection of UC of the UUT (reviewed in [8]). In our experience, FISH is very helpful in atypical UUT washing cytology. However, one needs to be familiar with tetraploid U-FISH patterns that may occur in reactive conditions in order to avoid false-positive FISH diagnoses, as discussed below. Examples of U-FISH in atypical urinary cytology are shown in Fig. 9.5.
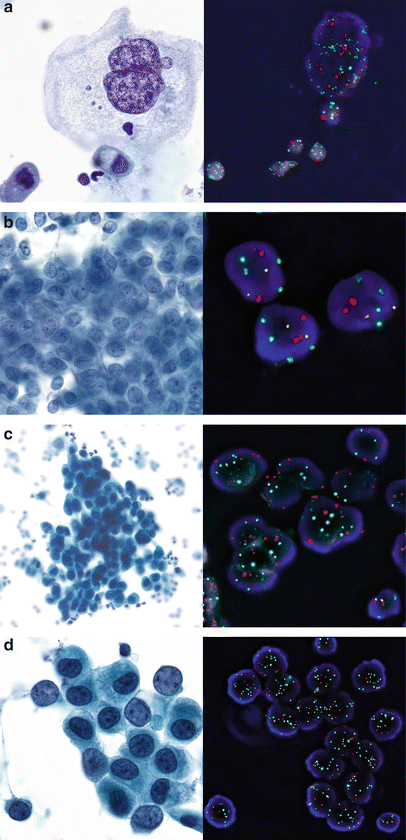

Fig. 9.5
Examples of UroVysion FISH and corresponding cytology (Papanicolaou stain) in urinary cytology. Chromosomes 3 in red, 7 in green, 17 in aqua and 9p21 in gold. (a) Benign bi- or multinucleate umbrella cell with pronounced reactive changes in a patient with irritativeFig. bladder (bladder washing). Highly increased number of all FISH signals due to repeated doubling of the genome (i.e., endoreplication), but no unbalanced copy number changes and no 9p21 deletion. (b) Sheets of mildly atypical urothelial cells in a washing of the renal pelvis. Normal FISH result (two copies per chromosome), consistent with reactive changes. (c) Atypical urothelial cells suspicious of high grade urothelial carcinoma (SHGUC) in a washing of the renal pelvis. Positive U-FISH result with increased copy numbers of all 3 chromosomes and a homozygous deletion of 9p21. Note the normal cell nucleus with retained 9p21 signals as internal normal control (right lower corner). (d) U-FISH-positive atypical urothelial cells (SHGUC) with increased copy number of all five chromosomes but no 9p21 deletion. Note the normal cell nucleus with normal copy numbers (right lower corner)
Cost-Effectiveness of UroVysion FISH
There have been concerns about the cost-effectiveness of the relatively expensive U-FISH testing, mainly because of the low positive predictive value. If applied to unselected patients with hematuria and recognizing the limited clinical value of enhanced detection of low grade UC, FISH results cost the patient a high price [8]. However, recent data suggest cost-effectiveness of negative U-FISH results following AUC results with equivocal or negative cystoscopy by avoiding unnecessary biopsies [13].
Pitfalls in UroVysion® FISH Analysis
Despite the evident utility of FISH in patients with AUC results, there is a possibility of false-positive results. Besides “anticipatory positive” FISH results, low specificity and low PPV in some studies might also be due to an increased number of tetraploid or dividing cells under reactive conditions. Although tetraploid cells are not specifically mentioned in the scoring guidelines of the manufacturer, it has now been recognized that rare cells with a tetraploid signal pattern (i.e., four signals of each probe) should not be taken as an unequivocally positive FISH result but interpreted with caution [3, 24–26]. In contrast, unbalanced numerical changes of one or more chromosomes (e.g., 2, 3, 5) or loss of 9p21 is virtually specific for neoplasia in bladder cytology. An exception is that pelvic irradiation (e.g., of the prostate or the uterus) often results in permanent chromosomal aberrations carrying a risk of a false-positive diagnosis by U-FISH. This is particularly the case for polysomies, whereas homozygous or heterozygous deletion of 9p21 remains highly specific for neoplasia even after irradiation. This emphasizes the need of appropriate clinical information and consideration of the typical postirradiation cytological changes. Decoy cells, i.e., polyoma-infected urothelial cells, can easily mislead by a false-positive cytological diagnosis of UC if one is not familiar with the morphological spectrum of decoy cells. Although there have been reports on rare FISH-positive decoy cells, most data suggest that polyomavirus infection does not interfere with U-FISH results [27].
Target FISH, in which there is cytological pre-screening of slides and cells in question prior to FISH, has been proposed as another way of making the FISH analysis more precise and specific by avoiding the analysis of obviously reactive bystander cells or the analysis of remaining material that might be devoid of the atypical cells and therefore not representative of the original slide [6, 24, 25]. This approach requires hybridization and scoring of the original stained slides and automated re-localization of the cells in question, ideally coupled with image-aided interpretation.
ImmunoCyt/uCyt+ and Other Cell-Based Tests
Several cytology -based tests other than DNA in situ hybridization have been developed to improve diagnostic accuracy of urinary cytology, especially its negative predictive value. To put the role of such markers into the right perspective, the performance of cytology is only low for LGUC but not for HGUC. Therefore, combining results of these tests is misleading.
Some of the tests have been approved for diagnostic use and surveillance by the FDA but none of them has been incorporated into recommended guidelines or clinical management [1, 28, 29]. Multiple studies have demonstrated that some urine markers are more sensitive than conventional urine cytology and there is also evidence that these tests may predict tumor progression and risk-stratify patients who are being treated with intravesical therapies [1, 28–32]. Data from meta-analyses suggest that cell-based assays may be more sensitive if compared to other assays especially in low grade tumors [29, 32]. This observation may be explained by the fact that current cell-based assays include markers for low grade tumors and are less confounded by urinary tract infections than liquid-based markers.
ImmunoCyt/uCyt+ Test
The uCyt test developed in 1997 [33] obtained FDA clearance in 2000 for the diagnosis and monitoring of bladder cancer. It combines three fluorescent monoclonal antibodies. Two of them (M344 and LDQ10), labelled with fluorescein (green fluorescence), have been raised against mucin-like antigens. The third one (19A211), labelled with Texas red (red fluorescence), recognizes a high molecular form of carcinoembryonic antigen. An example of a uCyt positive cytological specimen is shown in Fig. 9.6. The uCyt assay is technically simple and less expensive compared with U-FISH methodology and more sensitive in detecting low grade tumors [30]. Slides are read using an epifluorescent microscope with a dual-band filter specific to both fluorescein and Texas Red emission and excitation spectra. Samples are considered positive when at least one green or red fluorescent urothelial cell is observed. The antigens detected by M344 and 19A211 antibodies are expressed by 71 % and 90 % of Ta–T1 bladder tumors, respectively [34]. The usefulness of uCyt has been reported [28–31, 35–38] to detect both low grade and high grade cancers, including in situ carcinoma, as well as to predict recurrence in patients, all with a negative cystoscopy. This has been confirmed in an extensive meta-analysis [32]. The test may have a false-positive result due to inflammatory changes after BCG treatment but its overall sensitivity is high, ranging from 53 to 100 % (average 90 %), especially when combined with cytology (close to 100 %). Sensitivity increases with grade and level of invasion but its specificity is moderate to high (64–95 %; average: 74 %). uCyt improves the sensitivity and negative predictive value in detecting small, low grade tumors which makes this test well-suited for monitoring programs to decrease the frequency of follow-up cystoscopy [30]. However, despite being very promising, the test still needs to be validated in an independent large-scale prospective and multicenter study. Studies on cost-effectiveness are scarce [39].
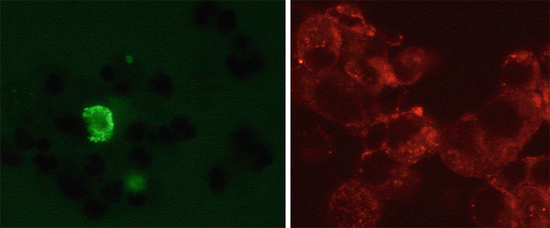

Fig. 9.6
Example of an ImmunoCyt/uCyt+ positive cytological specimen. Positive cells by red and green immunofluorescence (Texas red and fluorescein)
Other Cell-Based Tests
The ProEx C assay is another promising test consisting of a cocktail of antibodies, which detect both minichromosome maintenance protein (MCM2) and topoisomerase II-alpha protein (TOP2A) and in addition may help to stratify urine specimens difficult to classify morphologically [40, 41]. Other cytologic-based tests have been developed using single antibodies (Ki67, DD23), antibodies against Lewis X antigen, cytokeratin 20, or p16/Ki-67 dual-labelling [42]. A quantitative karyometric cytology system measuring nuclear shape and DNA content in light microscopic images has had variable results in terms of sensitivity and specificity [43]. Of note, almost all tumor markers previously mentioned have a much better sensitivity than cytologic examination but few reach the same level of specificity, and currently are not suitable for the clinical laboratory because of costs.
Liquid-Based Tests
Liquid- or non-cytology-based tests can be applied to voided urine samples either without the need for further preparation or on sediments of centrifuged urine samples. The two most commonly applied liquid-based urine tests on pure voided urine samples are the BTA™ (Polymedco Inc., Cortlandt Manor, NY, USA) and the NMP22™ (Bladder Check) test (Alere Inc., Waltham, MA, USA), both approved by the FDA, both for detection of bladder cancer in symptomatic patients and for monitoring of patients with a history of bladder cancer. For both tests a qualitative point of care test (BTA stat™ and NMP22-BladderChek™) and a quantitative version (NMP22™ and BTA TRAK™) are available. The latter tests require a dedicated laboratory with specialized personnel. The advantage of a point of care test is that it gives an immediate result and therefore can be done immediately prior to cystoscopy. A positive urine test may lead to a diagnostic review bias, which means that with the knowledge of a positive urine test result, the urologist may be more likely to detect a bladder neoplasm at cystoscopy [44]. These tests have been developed for both voided urine samples (not first-morning urine) and catheter-collected urines but not for barbotage fluids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


