Anatomy and Surgery of the Parotid Gland
Glenn E. Peters
Isaac A. Bohannon
J. Scott Magnuson
The paired parotid glands are the largest of the major salivary glands and are located in the preauricular region of the face. The facial nerve creates a division between the lateral and deep lobes of the gland, which is more surgical than anatomic in nature. The parotid is bounded posteriorly by the external auditory canal (EAC), superiorly by the zygoma, and inferiorly by the styloid process, the styloid muscles, as well as the internal carotid artery and the internal jugular vein. Also, a portion of the parotid gland extends posteroinferiorly over the mastoid tip and the sternocleidomastoid muscle, commonly known as the tail of parotid.
During the first 6 to 8 weeks of fetal development the major salivary glands develop. Outpouchings of oral ectoderm surrounded by mesoderm form the parotid anlage. As the anlage grows posteriorly, the facial nerve advances anteriorly toward the midline. Thus the facial nerve becomes surrounded by glandular tissue. Lymph nodes are encapsulated by the mesenchymal capsule surrounding the gland, creating intraparotid lymph nodes in both the lateral and deep lobes.
The parotid is surrounded by a continuation of the superficial layer of the deep cervical fascia. The thick superficial division of this fascial layer extends from the zygoma to the sternocleidomastoid, and the masseter anteriorly. The plane superficial to this fascia allows the surgeon to raise skin flaps without venturing too superficially into the subcutaneous tissues. This parotid capsule is very inelastic, and in the face of expansile masses or infectious processes can cause significant discomfort for patients.
The fascia deep to the deep lobe of the parotid forms the stylomandibular membrane; it is made up of the fascia of the posterior portion of the digastric muscle. This membrane divides the parotid space from the submandibular gland, spanning anteriorly from the mandible, inferiorly from the stylomandibular ligament, and posteriorly from the styloid process. When parotid masses herniate medially through the stylomandibular membrane, they can present as lateral pharyngeal wall masses as expansion continues in the parapharyngeal space.
Branches of the external carotid artery are the major blood supply to the parotid. It bifurcates into the superficial temporal and maxillary arteries at the level of the mandibular condyle. The transverse facial artery and vein, a branch of the superficial temporal vessels, travels between the parotid duct and the zygomatic arch to supply the parotid duct, masseter muscle, and parotid tissue.
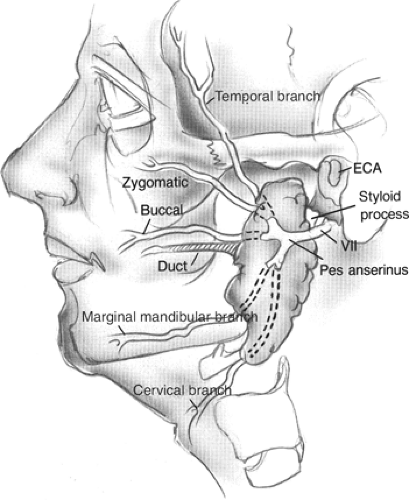
The retromandibular (posterior facial) vein is made up of the superficial temporal and maxillary veins. Located deep to the facial nerve, it is the dominant vein draining the parotid. The retromandibular vein is lateral to the carotid artery and exits the gland at the inferior aspect where it also joins the postauricular vein to make up the external jugular vein. It is also important to note that the retromandibular vein connects to the more anterior facial vein forming the common facial vein, which finally empties into the internal jugular vein (Figs. 1 and 2).
The parotid (Stensen’s) duct exits the gland on the anterior surface approximately 1.5 cm inferior to the zygoma. The duct courses 4 to 6 cm anteriorly to pierce the buccinator muscle. The papilla of the duct opens intraorally just opposite the maxillary second molar. Often there can be accessory glandular tissue along the duct. The buccal branch of the facial nerve usually follows a very close course with the duct.
The parotid gland is made up of a tubuloacinar system of ducts, which produces abundant watery saliva when stimulated by the parasympathetic nervous system. Once preganglionic parasympathetic neurons in the salivary nucleus of the brainstem synapse with autonomic ganglia they enter the parotid via their sensory nerves. The glossopharyngeal nerve (cranial nerve IX) supplies parasympathetic innervation to the parotid. Thus, the parasympathetic fibers are transported to the otic ganglion via the lesser petrosal nerve. Postganglionic fibers are then carried to the parotid gland by the auriculotemporal nerve (a branch of the cranial nerve V3).
The greater auricular nerve is encountered in elevating skin flaps. It is the largest branch of the cervical plexus and supplies sensation to the postauricular skin and the lobule of the ear. The nerve is often surgically divided as it passes over the posterior border of the sternocleidomastoid muscle.
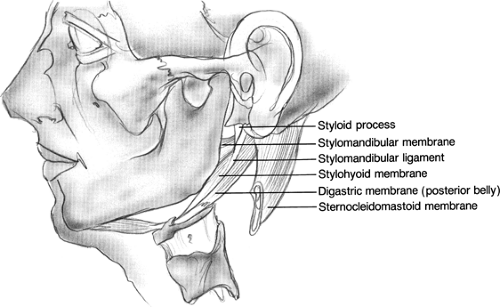 Fig. 2. Stylomandibular membrane (deep layer of parotid fascia). Herniations of parotid tissue through this membrane can result in a parapharyngeal mass. |
The facial nerve leaves the skull base through the stylomastoid foramen, which is located medial to the mastoid tip and lateral to the styloid process. As the nerve exits the foramen it gives off motor branches to the stylohyoid muscle, the posterior belly of the digastric, and postauricular muscles. The nerve then turns laterally to enter the parotid gland where it can be identified by the relationship to surrounding structures. The “tragal pointer” is a triangular extension of the cartilaginous EAC, which points toward the facial nerve. Generally, the nerve has been found 6 to 8 mm medial to the tympanomastoid suture line. In addition, the facial nerve is located inferiorly and just lateral to the styloid process, and is at the same level as the insertion of the posterior belly of the digastric muscle.
The main trunk of the facial nerve then splits at the pes anserinus (goose’s foot) into cervicofacial and temporofacial divisions. It should be recognized that considerable variability exists in the length of the main trunk and the branching pattern of the facial nerve as it courses through the parotid gland. Generally, the two divisions of the nerve branch again to form five major branches: temporal, zygomatic, buccal, marginal mandibular, and cervical. Intercommunicating branches are common between the major branches and should be preserved when possible.
During physical examination, palpation and bimanual manipulation of any parotid mass is critical. Examination of the lateral wall of the oropharynx may reveal a tumor of the deep lobe of the parotid or a parapharyngeal space mass. As with any mass, size, location, fixation to adjacent structures or skin, and quality of the mass can greatly contribute to treatment decision making and preoperative planning. Parotid masses can also present with facial nerve paralysis or weakness, which can be a herald of tumor invasion of the nerve. Nerve weakness should be characterized by the House–Brackmann scale of facial function.
Routine preoperative imaging of well-defined masses of the superficial parotid gland are unlikely to change the course of treatment. However, those tumors that raise clinical suspicions of malignancy, deep lobe, or parapharyngeal space origin should be evaluated with high-resolution imaging. The choice of imaging technique mainly depends on presumed pathology. Sialography, positron emission tomography, and technetium scanning are rarely used, having limited application to the majority of common salivary tumors. Ultrasonography has some application to identification of parotid abscesses, stones, or cysts, but is often used in conjunction with fine needle aspiration (FNA) biopsy. Computed tomography (CT) produces excellent images of the entire parotid gland, parapharyngeal space, mandible, temporal bone, and base of skull. CT is the most commonly used preoperative imaging tool. Alternatively, magnetic resonance imaging (MRI) has superior distinction of tumor, fat, and muscle based on different signal intensity. MRI is most often used to evaluate parapharyngeal space masses. The complementary strengths and weaknesses of MRI and CT sometimes mean that complex cases may need both imaging modalities to better understand the clinical picture.
FNA is known to be safe, simple to perform, and relatively inexpensive. Many studies have indicated that FNA has a high degree of sensitivity and specificity. Diagnostic accuracy tends to be better for benign rather than malignant tumors. The accuracy is most dependent on the experience of the cytopathologist and the volume of salivary tumors reviewed at the institution. Diagnostic error most commonly occurs with an inadequate sampling of the mass. Cell sampling can be improved by using ultrasonography in conjunction with FNA.
FNA is a common diagnostic practice in the head and neck region, but FNA of a parotid mass is a controversial issue. Is FNA really worth doing in the workup of salivary tumor? Will it change the course of treatment? Heller and colleagues demonstrated that only 35% of patients who underwent FNA had a change in their clinical approach to management of the tumor. Such changes included avoiding surgery in masses of inflammatory or lymphoma origin, and also simple observation in high-risk surgical patients with benign tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree