Anatomic Considerations in Gastroduodenal Surgery
Lee J. Skandalakis
David A. McClusky III
Marios Loukas
Petros Mirilas
B The Stomach and Duodenum
Introduction
The purpose of this chapter is to describe the embryology and anatomy of the stomach and duodenum—not in fine detail, but as it is approached in gastroduodenal surgery. An understanding of the anatomy flows from an understanding of the embryology.
The vast majority of gastroduodenal surgeries are still open and traditional. Laparoscopic surgery of the stomach and duodenum continues to evolve. Understanding the anatomic entities will allow the student and the experienced surgeon to transition easily from traditional surgery to a more minimally invasive approach when appropriate, and as supported by advances in device technology.
Embryology of the Stomach, Duodenum, Omenta, and Other Gastric Peritoneal Ligaments
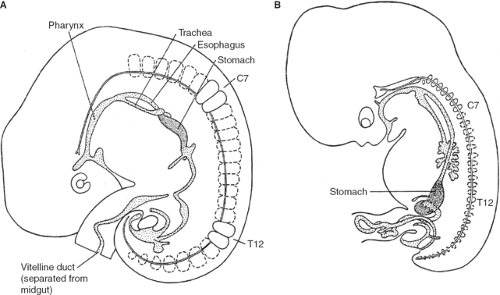
Below the tracheal diverticulum, the foregut of the embryo forms the esophagus, which extends until the dilation marking the developing stomach (Fig. 1). The gastric dilation is initially (5th week) positioned at the level of C3 to C5 vertebrae (Fig. 1A). With growth of the embryo, the foregut elongates; thus, the length of the esophagus increases and the stomach descends. At the end of the 7th week, the stomach is found between T11 and L4 (Fig. 1B).
The stomach and duodenum develop between the two folds of the mesentery. With reference to the intestine, the mesentery is divided into dorsal mesentery, which connects these splanchna to the posterior abdominal wall, and ventral mesentery, which connects them to the anterior abdominal wall (Figs. 2 and 3).
Other organs also develop in this configuration: the liver develops in the ventral mesentery and is connected to the anterior abdominal wall by the
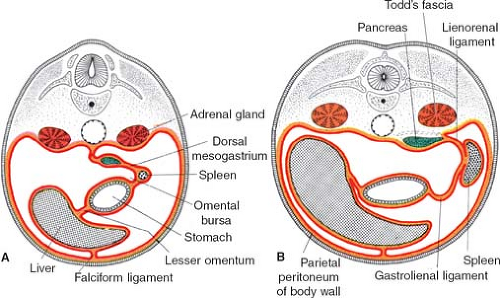
falciform ligament and to the stomach by the lesser omentum. In the dorsal mesentery the spleen and pancreas are found; the pancreas is behind the spleen. The peritoneal ligament between stomach and spleen is the gastrosplenic ligament, and then follows the ligament between spleen and pancreas and finally the ligament connecting the pancreas to the posterior wall.
falciform ligament and to the stomach by the lesser omentum. In the dorsal mesentery the spleen and pancreas are found; the pancreas is behind the spleen. The peritoneal ligament between stomach and spleen is the gastrosplenic ligament, and then follows the ligament between spleen and pancreas and finally the ligament connecting the pancreas to the posterior wall.
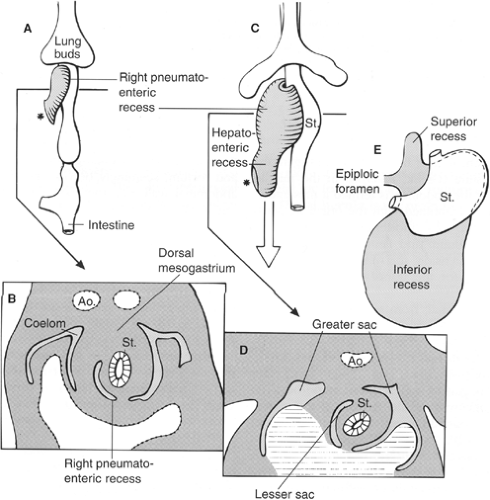
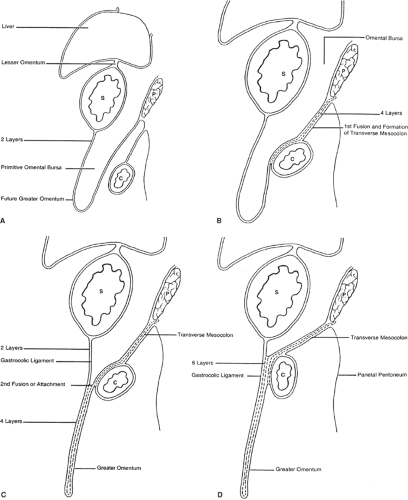
The initial development of these organs in a row (liver–stomach–spleen–pancreas) changes dramatically with the formation of the omental bursa, that is, the cavity behind the stomach. O’Rahilly and Müller point out that the omental bursa develops by the coalescence of small spaces and initially forms the right pneumatoenteric recess, between lung and stomach. The liver develops below this recess; the recess is associated with the liver and is named the hepatoenteric recess. Finally, the lesser sac develops behind the stomach, extends with a superior recess behind the liver, and with an inferior recess between the two leaves of the great omentum. From the beginning, the bursa has a communication with the rest of the abdomen, the omental foramen (Winslow’s) (Fig. 4).
The initial theories of embryologists were that a left turn of the stomach takes place and forms the omental bursa. These ideas were useful to understand the alteration of positioning of the vagus nerves: the left vagus on the anterior gastric wall, the right vagus on the posterior abdominal wall. However, such conjectures cannot explain the upper extension of the bursa around the caudate lobe of the liver or the opening of this cavity, the omental foramen (Winslow’s).
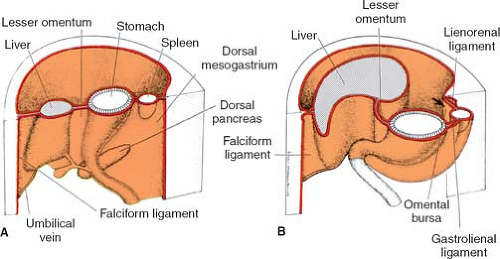
The truth about the “left turn” of the stomach relies on differential growth of the left wall of the stomach. This results in the formation of a lesser and greater curvature of the stomach, and the repositioning of the ventral and dorsal mesogastria (Figs. 2 and 3). Other major morphogenetic events are the growth and right-posterior location of the liver and the posterior positioning of the pancreas, which attaches to the posterior abdominal wall and becomes a secondarily retroperitoneal organ. The ligament attaching the pancreas to the posterior abdominal wall disappears and an avascular plane (Todd’s fascia) develops behind the pancreas (Fig. 2B). The remaining ligament connecting the spleen to the pancreas now becomes the splenorenal (lienorenal) ligament. The increased growth of the left side of the stomach also affects the respective dorsal mesogastrium, namely, the gastrosplenic ligament, which bulges downward and forms the omentum. The posterior double-layered leaf of the omentum attaches on the mesentery or the transverse colon; then, the two leaves of the omentum hanging down from the colon fuse between themselves (Fig. 5).
As a general rule, mesenteries connect organs to the abdominal wall and each other and carry all the vascular, neural, and lymph supply for the organs developing within them. Fulfilling this description of a mesentery, the lesser omentum will supply the pathway for the neurovascular supply of the liver, and will be the courier of the hepatic artery, portal vein, ducts, nerves, and lymphatics. The falciform ligament is the mesentery for the umbilical vessels reaching the hilum of the liver. The gastrosplenic ligament contains the short gastric arteries; the splenorenal ligament carries the splenic vessels. The vessels deriving from the aorta all lie in the retroperitoneum, where they course for a distance and then enter the respective mesenteries to reach the splanchna they supply. For instance, in the adult configuration, the hepatic artery courses retroperitoneally and then enters the lesser omentum, as does the portal vein. The right gastric artery also enters the lesser omentum to reach the lesser curvature.
The lower esophagus also has a mesentery, the mesoesophagus. After it passes the diaphragm the lower esophagus lies retroperitoneally; further caudad it regains its
mesentery, which is broad. The result is that the abdominal esophagus is covered by peritoneum anteriorly and on both sides laterally, while its posterior surface toward the diaphragm is still retroperitoneal, giving way to the entrance of the left gastric artery for the lower esophagus and the upper lesser curvature of the stomach.
mesentery, which is broad. The result is that the abdominal esophagus is covered by peritoneum anteriorly and on both sides laterally, while its posterior surface toward the diaphragm is still retroperitoneal, giving way to the entrance of the left gastric artery for the lower esophagus and the upper lesser curvature of the stomach.
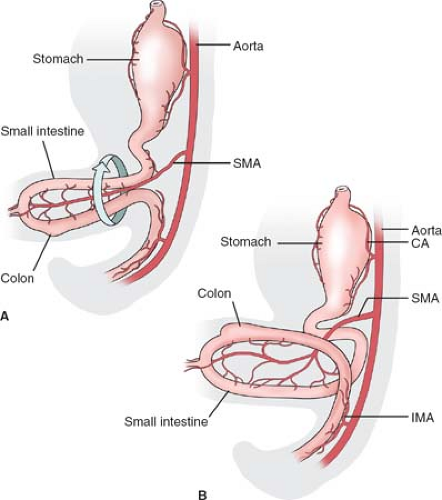
The adult duodenum corresponds to an area of the embryonic foregut and midgut distal to the stomach. The embryonic midgut undergoes a normal herniation into
the umbilical cord during the 5th week of gestation (Fig. 6A). This part of the intestine is fed by the superior mesenteric artery (SMA). The herniated intestine undergoes a rotation of 90 degrees around the axis of this artery, bringing the colon over the small intestine. In the 10th week intestines return in the abdomen. The duodenum comes back first and takes its position under the SMA, while the cecum is still in the umbilical cord (Fig. 6B). The distal ileum and the colon are positioned in front of the SMA, and during their return they undergo another 180-degree rotation. Knowledge of these relations is essential for the surgeon in order to understand the normal anatomy, and the cases of anomalies of rotation affecting the duodenum as well as the possible compression of the duodenum under the SMA.
the umbilical cord during the 5th week of gestation (Fig. 6A). This part of the intestine is fed by the superior mesenteric artery (SMA). The herniated intestine undergoes a rotation of 90 degrees around the axis of this artery, bringing the colon over the small intestine. In the 10th week intestines return in the abdomen. The duodenum comes back first and takes its position under the SMA, while the cecum is still in the umbilical cord (Fig. 6B). The distal ileum and the colon are positioned in front of the SMA, and during their return they undergo another 180-degree rotation. Knowledge of these relations is essential for the surgeon in order to understand the normal anatomy, and the cases of anomalies of rotation affecting the duodenum as well as the possible compression of the duodenum under the SMA.
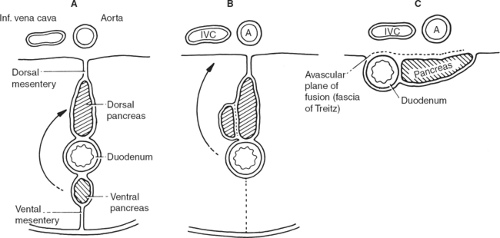
As pointed out previously, abdominal splanchna develop in the mesentery; so does the duodenum, which initially has a mesentery (mesoduodenum). The ventral pancreas also develops dorsally in the mesoduodenum and is met by the ventral pancreas, by way of the
“left turn” of the stomach, also affecting this lower area (Fig. 7). The duodenum and the pancreas finally locate retroperitoneally (secondarily retroperitoneal organs). The right leaf of the mesentery becomes posterior, meets the parietal peritoneum of the dorsal abdominal wall, and fuses with it, forming an avascular plane. The surgeon who knows this can approach from the left (where no mesentery is found and consequently no vessels exist), dissect and detach the duodenum together with the head of the pancreas, and recreate the original mesentery with all vessels in hand, without the risk of bleeding (Kocher’s maneuver).
“left turn” of the stomach, also affecting this lower area (Fig. 7). The duodenum and the pancreas finally locate retroperitoneally (secondarily retroperitoneal organs). The right leaf of the mesentery becomes posterior, meets the parietal peritoneum of the dorsal abdominal wall, and fuses with it, forming an avascular plane. The surgeon who knows this can approach from the left (where no mesentery is found and consequently no vessels exist), dissect and detach the duodenum together with the head of the pancreas, and recreate the original mesentery with all vessels in hand, without the risk of bleeding (Kocher’s maneuver).
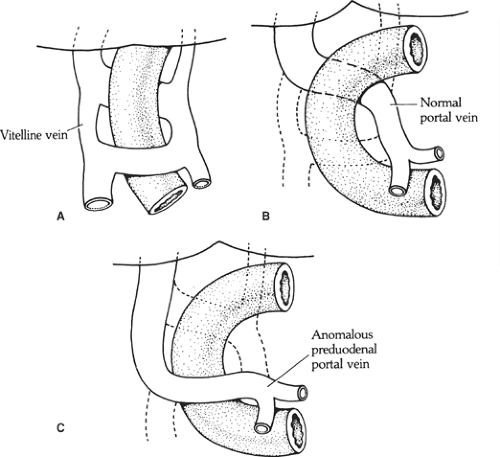
Another aspect of embryology necessary to understand the anomaly of a preduodenal portal vein is that the vitelline (omphaloenteric) veins, which follow the intestine of the embryo, undergo a major reconstruction of communications between them to form the portal vein (Fig. 8). Normally, the anterior communication disappears and the posterior stays; a portal vein thus formed would locate behind the duodenum. In the opposite case (the anterior, lower communication is kept and the posterior, upper one disappears), a preduodenal portal vein is formed.
A last point of embryologic consideration for the surgeon is that the embryogenesis of the lymphatics follows the arteries. This is extremely important to remember and applies everywhere. All lymph nodes are encountered along arteries; nodes and the end of the lymph flow lie at the stem of the arteries. Since the mother artery of the upper abdomen is the celiac trunk, the final destination of lymph flow will be the celiac nodes. The nerves also follow the arteries to reach the respective splanchna. The formation of arteries is a strong morphogenetic event; veins are not the guide for lymphatics and nerves.
Table 1 Anomalies of the Stomach
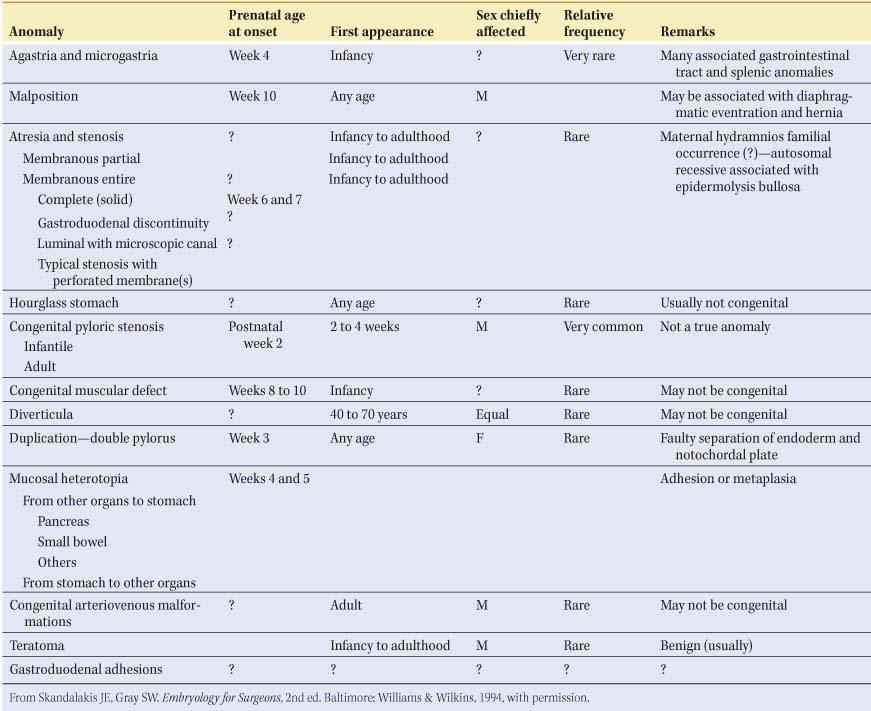 |
Congenital Anomalies of the Stomach and Duodenum
Duodenum
Malrotation and nonrotation: The duodenum is not located under the SMA; the duodenojejunal flexure is absent. In incomplete (“mixed”) rotation the prearterial segment has failed to rotate; the congenitally high cecum lies in front of the duodenum, where it is fixed to the posterior abdominal wall and may compress the duodenum (Fig. 9A). In nonrotation, the postarterial part (distal ileum or colon) of the midgut returns first; the colon is found on the left and the small intestine on the right (Fig. 9B).
Duodenal stenosis and atresia: anomalies caused by failure of recanalization of the lumen of the duodenum, which is normally occluded between the 4th and 7th week by proliferation of the epithelium, followed by recanalization. Stenosis of the duodenum may be associated with annular pancreas or with aberrant pancreatic tissue in the duodenal wall.
Duodenal diverticula: usually on the concave (i.e. pancreatic) wall of the second and third part of the duodenum. They are usually solitary and asymptomatic. O’Rahilly suggests that they may be caused by persistence of normally occurring transitory diverticula that are present in the embryo.
Preduodenal portal vein: caused by abnormal formation of the portal vein as described above (Fig. 8). The vein may compress the duodenum to the point of obstruction.
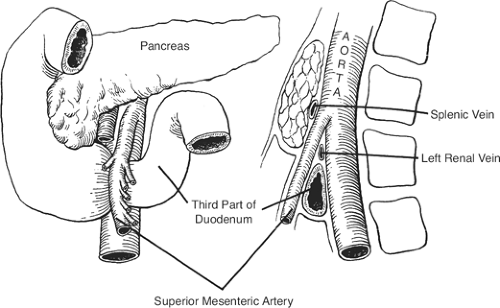
SMA syndrome: the result of the compression of the third part of the duodenum between the superior mesenteric and the aorta (Fig. 10). The condition may be caused by a short mesentery of a congenitally narrow angle between the artery and the aorta.
Gross Anatomic Parts of the Stomach and Duodenum
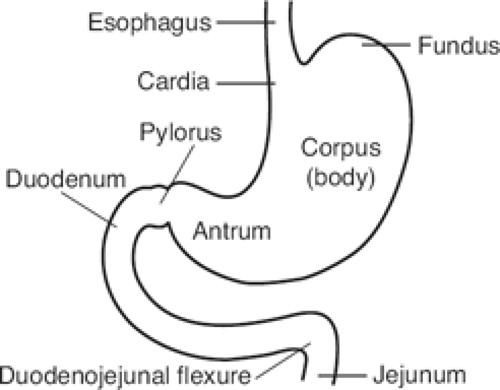
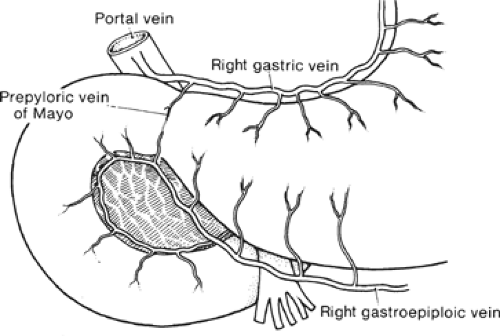
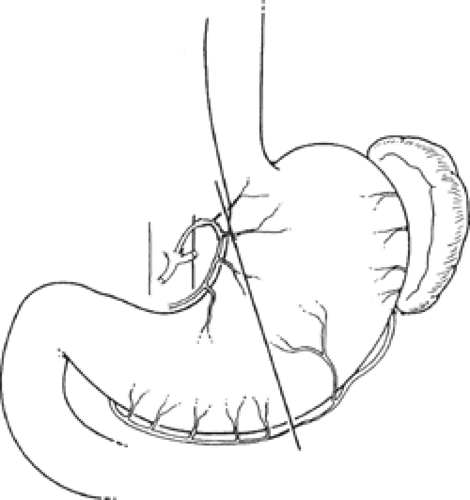
The gross anatomic parts of the stomach are the cardia, the fundus, the body, the pyloric antrum (which is the proximal part of the pylorus), and the pyloric canal (Fig. 11). There is no external demarcation between the cardia and the fundus of the stomach. The fundus is considered as the part of the stomach located above the entrance of the esophagus. The body of the stomach is the part between the fundus and the pylorus. Again, there is no external demarcation between the body and the fundus. A fairly consistent landmark is an angular notch found two-thirds of the way down the lower curvature, which delimits the body from the pyloric portion; a slight constriction at the end of the pyloric portion marks its junction with the duodenum (Fig. 12). The junction of the body of the stomach with the antrum can be estimated as in Figure 13. While an external examination provides no clue to the line of junction between the stomach and the duodenum, some surgeons use the so-called pyloric vein as their guide (Fig. 12).
The duodenum has an upper, a descending, a horizontal, and an ascending part. The first, or free, part is not attached to the posterior abdominal wall. This part is attached to the hilum of the liver by the hepatoduodenal ligament. It is covered by peritoneum from the front and behind, unlike the remaining parts of the duodenum, which are retroperitoneal.
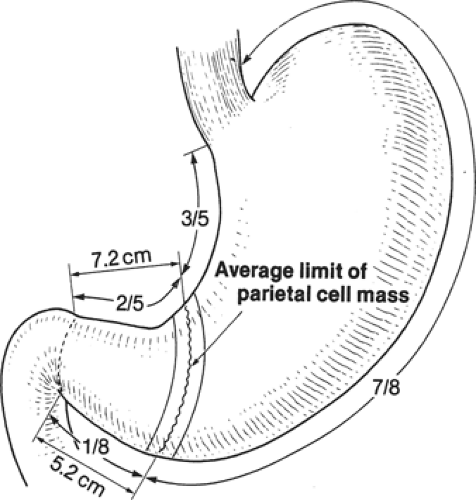
From a surgical standpoint, the stomach can be divided into two almost separate organ systems or “gastric units,” each with its own special anatomy, pathology, and surgical significance. The proximal gastric unit consists of the gastroesophageal junction and the proximal stomach (Fig. 14). The distal gastric unit includes the gastric antrum, pylorus, and the first part of the duodenum (Fig. 15). The border between the proximal and distal stomach is an arbitrary line from the lesser curvature to the greater curvature (Fig. 16). This may be thought of as (a) a line starting on the lesser curvature at the third vein down from the gastroesophageal junction to the greater curvature at the point where the left gastro-omental vessels come closest to the gastric wall, or (b) a line starting on the lesser curvature at the first descending branch of the left gastric artery to the greater curvature at the midpoint of the left gastro-omental artery.
Surgicoanatomic Units of the Gastroduodenum
Surgically, the gastroesophageal and gastroduodenal junctions are perhaps the most difficult anatomic units in the operating room.
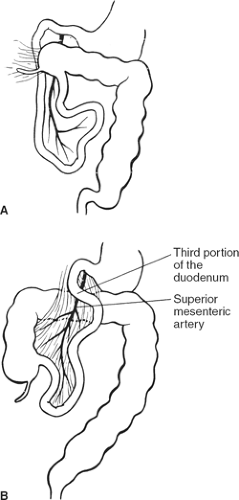 Fig. 9. A: Mixed rotation. B: Nonrotation. (From Skandalakis JE, Gray SW, Rowe JS Jr. Anatomic Complications in General Surgery. New York: McGraw-Hill, 1983.) |
Gastroesophageal Junction
There are several anatomic entities related to the gastroesophageal area such as the diaphragm, distal esophagus, proximal gastric unit, lesser omentum, gastrophrenic ligament, and gastrosplenic ligament.
The esophagus joins the stomach in the abdomen, just below the diaphragm. The gastroesophageal junction is defined as the area where the distal esophagus joins the proximal stomach (cardia).
In its passage through the esophageal hiatus, the esophagus is bounded by the two diaphragmatic crura and the phrenoesophageal membrane (Fig. 17). The length of the abdominal esophagus is from 0.5 to 2.5 cm, but the surgeon has access to an appreciable length of esophagus below the diaphragm.
The abdominal esophagus is related to surrounding structures:
Anterior: Posterior surface of left lobe of liver
Posterior: Right crus of diaphragm, aorta
Right: Caudate (spigelian) lobe of liver
Left: Fundus of stomach
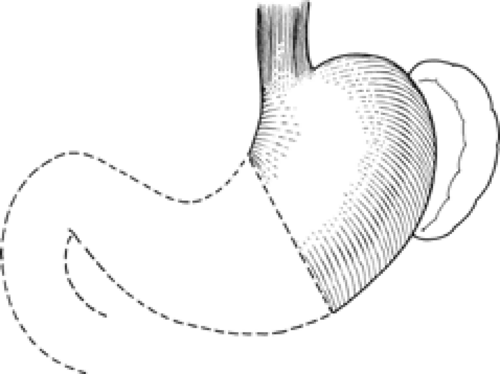 Fig. 14. The proximal gastric surgical unit. (From Skandalakis JE, Gray SW, Rowe JS Jr. Anatomical Complications in General Surgery. New York: McGraw-Hill, 1983.) |
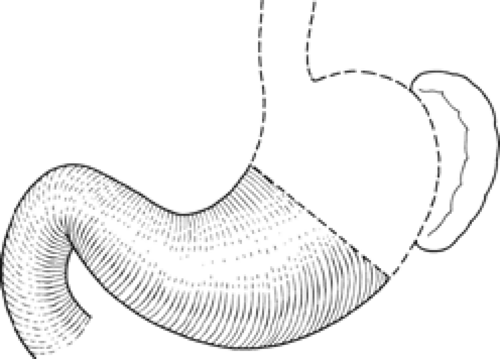 Fig. 15. The distal gastric surgical unit. (From Skandalakis JE, Gray SW, Rowe JS Jr. Anatomical Complications in General Surgery. New York: McGraw-Hill, 1983.) |
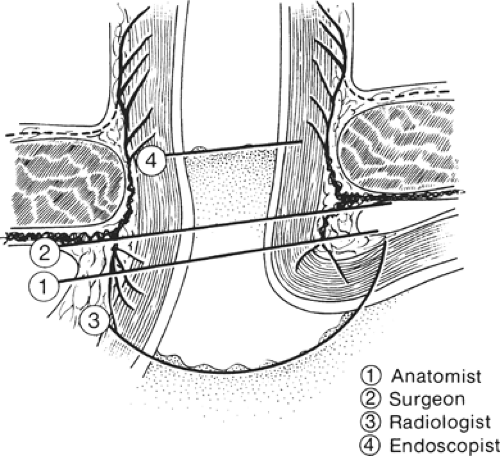
Because the histologic junction between the esophagus and stomach is not coincident with the external junction, the “gastroesophageal junction” differs with each specialist (e.g., anatomist, surgeon, radiologist, and endoscopist) (Fig. 18). The histologic junction is marked by an irregular boundary between the stratified squamous epithelium and the columnar epithelium. The loose submucosal connective tissue allows the mucosa to move freely over the muscularis, bulging in folds into the stomach at deglutition. The junctional level may change even at rest, being lower in the full stomach than in the empty one.
The columnar epithelium forms a mucosa unlike that of the body of the stomach. Its glands, lacking chief and parietal cells, secrete mucus. Histologists refer to these as cardiac glands. “Junctional epithelium” has been proposed as a name for this area between typical esophageal and typical gastric mucosae (Fig. 19). According to Odze, “The true gastric cardia is an extremely short
segment (less than 0.4 mm) of mucosa that is typically composed of pure mucous glands, or mixed mucous/oxyntic glands that are histologically indistinguishable from metaplastic mucinous columnar epithelium of the distal esophagus. In patients with gastroesophageal reflux disease, whether physiologic or pathologic, the length of cardia-type epithelium increases and extends proximally above the level of the anatomic gastroesophageal junction into the distal esophagus.”
segment (less than 0.4 mm) of mucosa that is typically composed of pure mucous glands, or mixed mucous/oxyntic glands that are histologically indistinguishable from metaplastic mucinous columnar epithelium of the distal esophagus. In patients with gastroesophageal reflux disease, whether physiologic or pathologic, the length of cardia-type epithelium increases and extends proximally above the level of the anatomic gastroesophageal junction into the distal esophagus.”
The gastroesophageal junction has been found to be a highly competent barrier to reflux of gastric content into the esophagus. While the lower esophageal sphincter may be the most important component of the barrier, a fold of muscle and mucosa created by the angle of entry of the esophagus into the stomach (the so-called gastroesophageal flap valve) also contributes to the closing mechanism.
A major pathophysiologic effect of hiatus hernia is gastroesophageal reflux, which contributes to esophageal mucosal injury, most notably in patients with severe gastroesophageal reflux disease. Together with the crural diaphragm, the lower esophageal sphincter functions as antireflux barrier, protecting the esophagus from caustic gastric content. Achalasia and gastroesophageal reflux disease are two typical examples of dysfunction of the lower esophageal sphincter. In the presence of normal anatomy of the gastroesophageal junction (i.e., absence of hiatus hernia), the pressure of the gastroesophageal junction is the most important determinant of the strength of the antireflux barrier.
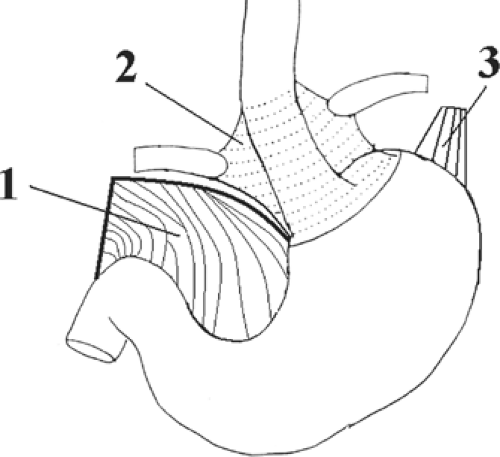 Fig. 17. Anchoring structures of the endoabdominal esophagus. The diaphragmatic crura are not shown. 1. lesser omentum (cut), 2. phenoesophageal membrane, 3. gastrophrenic ligament. |
Gastroduodenal Junction
The first part of the duodenum has the following location, pathway, and relations: It is located in front and to the right of the spinal column and the inferior vena cava and is not fixed with other anatomic entities of the posterior abdominal wall. Behind, it is related to the liver, the gallbladder, the bile duct, portal vein, pancreatic head, and pancreatic neck. The first part of the duodenum has a length of approximately 5 cm; its proximal half is mobile and its distal half is fixed. The duodenum is responsible for anchoring the distal gastric unit; together they form the gastroduodenal junction. Three histologically distinct types of normal duodenal mucosa are found at the gastroduodenal junction: antral, transitional, and jejunal.
In contradistinction to most gut sphincters, the human pylorus maintains a patent lumen most of the time, only intermittently becoming a tightly closed barrier arresting transit to and from the stomach. Contraction of its proximal and distal muscle loops and occlusion of its lumen by mucosal folds mediates pyloric closure.
The following points need to be remembered:
There is a circular muscle at the pylorus that closes with every contraction of the gastric antrum.
The pylorus contracts when the duodenum contracts in order to prevent the reflux of bile salts.
The parasympathetics (vagus nerve) enhance gastric wall contractions.
The sympathetics inhibit contractions.
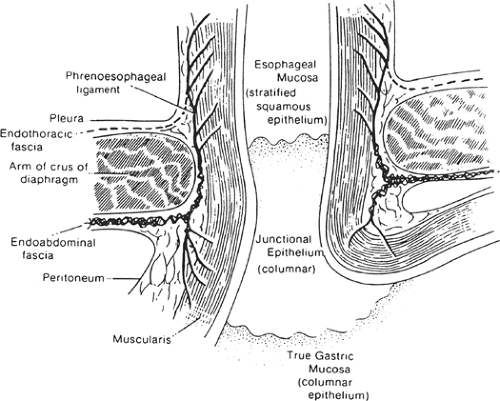 Fig. 19. Coronal section through gastroesophageal junction and esophageal hiatus of diaphragm.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|