http://evolve.elsevier.com/Edmunds/NP/
 Top 100 drug;
Top 100 drug;  key drug. Morphine has been designated a key drug because, traditionally, all opioids were compared with morphine.
key drug. Morphine has been designated a key drug because, traditionally, all opioids were compared with morphine.
Information about acetaminophen can be found in Chapter 35.
Information about aspirin and NSAIDs can be found in Chapter 36.
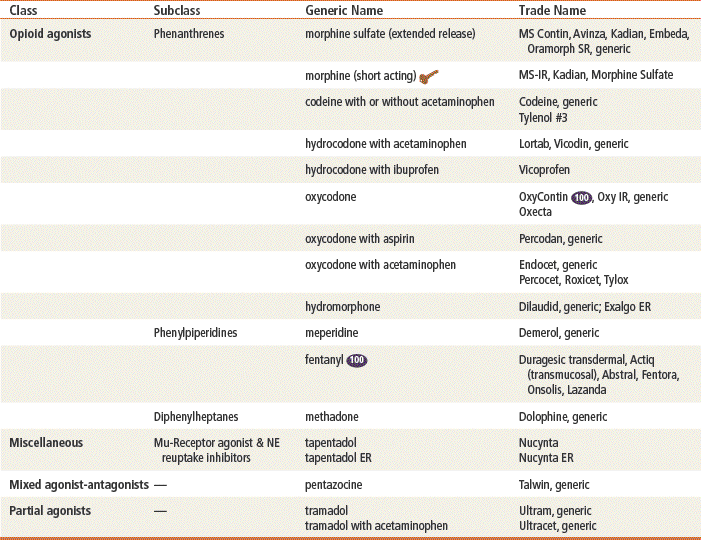
This chapter discusses pain management in the outpatient setting, including the use of drugs other than opioids, but it focuses on the use of opioids in pain management; only the common drugs listed in the previous table are discussed in detail.
Natural opioids come from opium, which is obtained from unripe seed capsules of the poppy plant. Opium contains more than 20 distinct alkaloids. The main phenanthrenes are morphine, codeine, and thebaine. The benzylisoquinolines are papaverine (a smooth muscle relaxant) and noscapine. Heroin is diacetylmorphine, which is metabolized to morphine.
Synthetic opioids were developed in the hope of producing a less addictive drug. These attempts failed, but the drugs have proved to be extremely useful for management of both acute and chronic pain. Many semisynthetic derivatives are made through simple modifications of morphine or thebaine. Morphine is the precursor of the synthetic opioid analgesics hydrocodone, hydromorphone, and oxycodone. Thebaine is the precursor of naloxone, an opioid antagonist (see Chapter 50). The phenylpiperidines and the diphenylheptanes are chemical classes that are structurally distinct yet similar to morphine. These drugs have actions similar to those of morphine.
The opioids are classified as narcotics and thus are considered controlled substances because of their abuse potential, as mandated by the Controlled Substances Act of 1970. Clinicians should be familiar with regulations regarding the use and dispensing of narcotic analgesics. Methadone is prescribed chiefly for the treatment of opiate detoxification and may be dispensed only by pharmacies and maintenance programs approved by the FDA and by state authorities, according to the requirements of the Federal Methadone Regulations. Methadone also is used for pain management; it may be prescribed by any provider with a DEA license to prescribe Schedule II drugs and can be dispensed by any licensed pharmacy (see Chapter 10). Because of the potential for abuse, the health care provider must justify the use of opioid analgesics for ambulatory patients.
Morphine is the standard opioid with which all others are compared. It is used extensively in acute care and hospice settings. Codeine, hydrocodone, and oxycodone are used frequently in combination with acetaminophen in both acute and primary care settings. Hydromorphone is very potent (five times the potency of morphine) and is reserved for severe pain not relieved by morphine; primary care providers generally will not prescribe this drug. Opioid agonist-antagonists also are used and may be preferred over traditional opioids for use in ambulatory patients because their potential for abuse is lower. Pentazocine has limited use because of CNS toxicity. Tramadol is a weak opioid agonist that inhibits the reuptake of norepinephrine and serotonin, thus modifying the patient’s perception of pain.
Increasing numbers of newborns suffering drug withdrawal symptoms led the American Academy of Pediatrics to update treatment guidelines and call on hospitals to develop drug abuse screening protocols for mothers.
Therapeutic Overview
Melzack and Wall’s gate control theory of pain is the most comprehensive pain theory proposed to date. They suggest that four processes are required for pain to occur: transduction, transmission, modulation, and perception. Sensory receptors, or nociceptors, that are sensitive to painful or tissue-damaging (noxious) stimuli are present in the skin, bone, muscle, connective tissue, and thoracic, abdominal, and pelvic viscera.
Transduction occurs when a noxious stimulus depolarizes peripheral nerve endings and sets off electrical activity. The nerve endings that transduce the noxious stimuli conduct electrical signals to the spinal cord through two types of nerve fibers: A delta fibers and C fibers. A delta fibers are myelinated, and their activation is associated with sharp, stinging sensations. C fibers are unmyelinated, and their activation is associated with vaguely localized pain that may be dull or burning.
Next, transmission occurs, whereby electrical impulses are carried throughout the peripheral and CNS. Modulation is the central neural activity that controls the transmission of pain impulses. Finally, during perception, the neural activities involved in transmission and modulation result in a subjective correlate of pain that includes behavioral, psychologic, and emotional factors (Figure 43-1).
Disease Process
Pain is defined by the International Association for the Study of Pain (IASP) as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage. Pain is always subjective.”
Opioid Tolerance, Dependence, and Addiction
All opioid drugs cause tolerance and dependence. This is not the same as abuse. Tolerance is a pharmacologic phenomenon characterized by decreasing drug effect over time. More drug is needed to produce the same effect. Dependence is the physiologic development of abstinence syndrome or withdrawal symptoms when a drug is discontinued or an antagonist is given. Slowly tapering the drug can eliminate withdrawal symptoms. Psychologic dependence, or addiction, is the overwhelming obsession with obtaining and using a drug for a non–medically approved purpose. Tolerance and dependence are the consequences of regular use of an opioid for a particular length of time and are not a problem. Addiction is a problem; however, a patient in pain should not be deprived of adequate pain relief because of fear of addiction.
Some patients exhibit specific behaviors when pain is undertreated; this has come to be called pseudoaddiction. These individuals watch the clock, demand pain medication, and may even resort to illegal means to get drugs. This phenomenon can be distinguished from true addiction in that the behavior ceases when the patient attains adequate pain control.
Origin of Pain
Although the trend is to consider pain as a single entity, some authors still distinguish between acute and chronic pain. Acute pain usually is related to an identifiable injury, such as recent surgery, trauma, or infection, and resolves within a predictable and expected time interval. Chronic pain seems to be the result of physiologic changes in the nervous system caused by untreated or undertreated, persistent acute pain. Although injury may initiate chronic pain, factors remote from its cause may perpetuate it, leading to unexplainable persistence. Currently, an estimated 50 million Americans suffer from chronic pain. This number will increase as the population ages and treatment for pain-related chronic health problems, such as back disorders, degenerative joint diseases, rheumatologic conditions, visceral diseases, and cancer, is increased. In the person with cancer-related pain, about 60% to 70% of pain is related to the tumor, 15% to 20% is related to diagnostic procedures or treatment for cancer, and the remaining 0% to 15% is completely unrelated to cancer or its treatment.
Classification of Pain
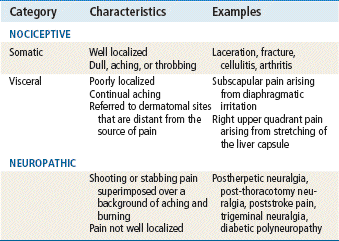
Pain can be classified as nociceptive or neuropathic (Table 43-1). Nociceptive pain is further divided into two categories: somatic and visceral. Nociceptive pain arises from the direct stimulation of afferent nerves in cutaneous or deep musculoskeletal tissues. It occurs in response to tissue injury or disease infiltration of the skin, soft tissue, or viscera. Somatic pain is well localized in skin and subcutaneous tissues but not in bone, muscle, blood vessels, and connective tissue and is often described as dull or aching. Visceral pain is poorly localized and often is described as a continual aching, or a deep, crampy, or sharp, squeezing pain. Visceral pain may be referred to dermatomal or myotomal sites that are distant from the source of pain. Visceral pain occurs in response to stretching, distention, compression, or infiltration of organs such as the liver.
Neuropathic pain, which results from injury to peripheral nerves or to the CNS, is described as episodes of shooting or stabbing pain superimposed over a background of aching and burning. Because it originates from peripheral nerves, spinal cord, or brain, it is poorly localized and often is associated with paresthesias and dysesthesias.
Assessment
It is important for the clinician to determine the cause of pain whenever possible. Even in a patient with terminal cancer, each new pain should be evaluated for a proximate cause that may be specifically treated. For example, bone pain may be ameliorated by radiation. Severe flares of pain are called “breakthrough pain” because they may break through the pain medication. Patients learn what triggers these breakthrough episodes so they can plan with the provider for how to prevent or resolve them.
Initial pain assessment seeks to characterize the pathophysiology of the pain, identify its cause, determine its intensity, and evaluate its impact on the patient’s ability to function. Assessment should include a detailed history and physical examination, psychosocial and functional assessment, and diagnostic evaluation as indicated. Associated findings of acute pain can include facial grimacing, tachycardia, hypertension, pallor, diaphoresis, mydriasis, and nausea. Chronic pain may be accompanied by fatigue, depression, sleep disturbance, decreased appetite, increased irritability, and decreased libido. Visible facial expressions of pain usually are lacking, and physiologic signs may be absent. Patients also can have intermittent chronic pain, such as recurrent episodes of neuralgia, headache, or angina.
Because pain is a subjective experience, assessment also must include patient self-report, which includes a description of pain and its onset, duration, and diurnal variation, as well as information on the location and radiation of pain, its intensity or severity, aggravating and relieving factors, and the patient’s goal for pain control. A variety of common pain assessment tools can be used to help grade pain severity in children and adults.
Follow-up assessment of the outcome of pain management is required to assess the effectiveness of the intervention. Changes in pain pattern or the development of new pain should trigger reassessment, diagnostic evaluation, and modification of the treatment plan. Documentation of a patient’s adherence with regard to dosing and duration of prescriptions is essential for all pain management. The provider should assess functioning and barriers to or facilitators of nonprescription treatment, especially sleep, activity, diet, diversion, and so forth.
The Agency for Health Care Policy and Research (AHCPR) clinical practice guidelines, although dated, include the following classic principles of pain assessment (A-A-B-C-D-E-E):
Mechanism of Action
Opioid analgesics are thought to inhibit painful stimuli in the substantia gelatinosa of the spinal cord, brainstem, reticular activating system, thalamus, and limbic system. Opiate receptors in each of these areas interact with neurotransmitters of the autonomic nervous system, producing alterations in reaction to painful stimuli. The opioid action of the drug manifests as analgesia, sedation, euphoria, mental clouding, respiratory depression, miosis, decreased peristaltic motility, depression of the cough reflex, and orthostatic hypotension.
Opiate receptors in the CNS mediate analgesic activity. Opioid agonists occupy the same receptors as endogenous opioid peptides, and both alter the central release of neurotransmitters from afferent nerves sensitive to noxious stimuli.
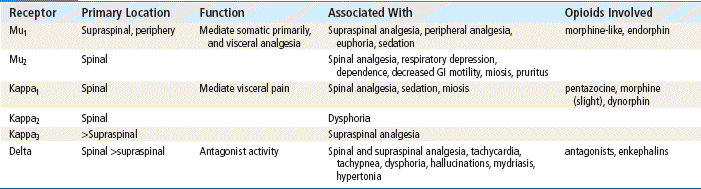
Actions of opioid analgesics can be defined by their activity at three specific receptor types: mu, kappa, and delta. The mu receptors mediate morphine-like supraspinal analgesia, miosis, respiratory depression, euphoria, physical dependence, and suppression of opiate withdrawal. The kappa receptors mediate spinal analgesia, respiratory depression, and sedation. The delta receptors mediate antagonist activity (Table 43-2). Morphine-like agonists have activity at the mu, kappa, and delta receptors. Mixed agonist-antagonist drugs, such as pentazocine, have agonist activity at some receptors and antagonist activity at other receptors. The opioid antagonist naloxone does not have agonist activity at any opioid receptors.
The mechanism by which opioids produce euphoria is not clear. They alter hypothalamic heat regulation in such a way that body temperature often falls slightly. They also affect the hypothalamus by causing decreased levels of testosterone, cortisol, ACTH, and β-endorphin. With long-term use, this effect diminishes. CNS effects in the limbic system can include dysphoric mood, especially with long-term use, intense and unusual dreams, and hallucinations. Pupillary miosis results from excitatory effects on the parasympathetic nerves that cause constriction of the pupil.
The respiratory depression associated with opioids is caused by a direct effect on the brainstem respiratory centers by which the brainstem becomes less responsive to carbon dioxide. Death from morphine overdose is usually the result of respiratory arrest. The cough reflex is depressed by a direct effect on the cough center in the medulla. This event is separate from respiratory depression.
Opioids produce nausea and vomiting through direct stimulation of the chemoreceptor trigger zone for emesis in the medulla. A vestibular component is part of the effect, in that nausea and vomiting are more common in ambulatory patients than in those on bed rest.
Opioids produce peripheral vasodilation, reduce peripheral vascular resistance, and inhibit baroreceptor reflexes, causing orthostatic hypotension and fainting.
The effects of opioids on the GI system are many. They decrease gastric motility, thereby prolonging gastric emptying time and increasing the likelihood of esophageal reflux. With opioid use, the absorption of other orally administered drugs is retarded. Opioids diminish biliary, pancreatic, and intestinal secretions. The sphincter of Oddi constricts and causes increased pressure in the common bile duct, leading to biliary colic. Opioids delay digestion of food in the small intestine. Propulsive peristaltic waves in the colon are diminished and tone is increased to the point of spasm. This delays the passage of stool and leads to constipation.
Opioids inhibit the urinary voiding reflex and may cause urinary retention. They also may prolong labor. Opioids cause dilation of cutaneous blood vessels, resulting in flushing. Some opioids—morphine and meperidine, but not methadone or fentanyl—cause histamine release. This may lead to pruritus, sweating, and urticaria. The opioids affect the immune system in a manner that is poorly understood, but they seem to cause suppression of natural killer cells.
Mixed Agonist-Antagonists
The mixed agonist-antagonists produce potent analgesic effects by stimulating the kappa receptor and blocking the mu receptor. Thus, they may produce withdrawal symptoms in patients with opioid dependency but are also less likely to be abused when compared with pure opioid agonists.
In addition to central opiate receptor agonist activity, tramadol exerts norepinephrine and serotonin reuptake inhibition in the CNS, which inhibits pain transmission in the spinal cord.
Norepinephrine Reuptake Inhibitor
Tapentadol is a new synthetically derived, centrally acting oral analgesic. In addition to central opiate receptor agonist activity, it activates the mu-opioid receptor and inhibits norepinephrine synaptic reuptake. Norepinephrine reuptake inhibition appears to have an additive analgesic effect to that of the drug’s opioid activity.
Treatment Principles
Evidence-Based Recommendations
Cardinal Points of Treatment
Nonpharmacologic Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree