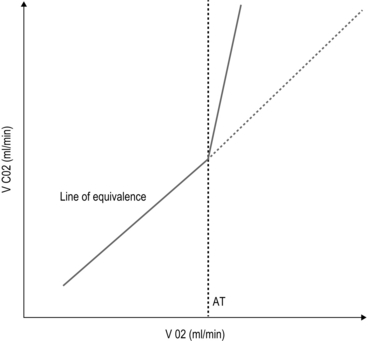
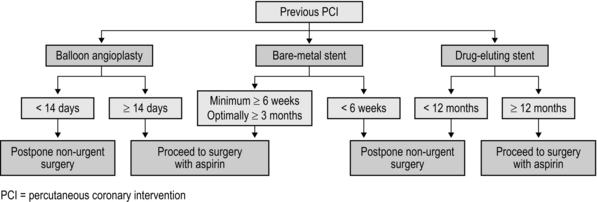
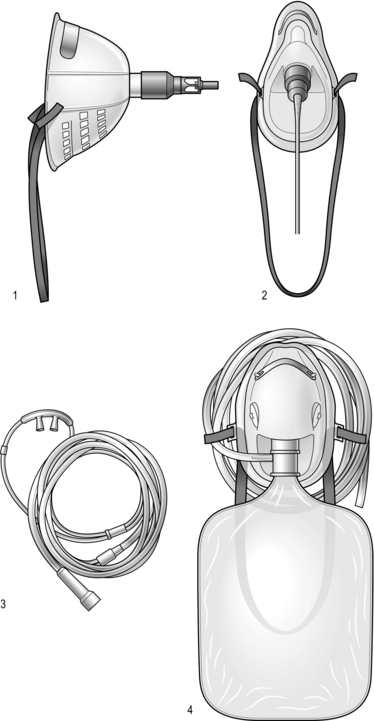
2 The first widely used cardiac risk index was that proposed by Goldman et al in 1977.1 Nine independent criteria were identified as indicators of increased risk (Box 2.1). The Goldman Index has been revised by subsequent workers, notably Detsky2 and Lee.3 In 2007, the American College of Cardiology (ACC) and American Heart Association (AHA)4 sought to stratify apparent cardiac risk factors into three categories – those that require further investigation, and others that may or may not actually impose increased risk (Box 2.2). Subsequent guidelines propose a stepwise approach to the evaluation of a potential high-risk surgical patient. The aim is to assist in creating an individualized cardiac risk assessment, and to suggest appropriate interventions before surgery in terms of optimization. The process is summarized in Box 2.3 and expanded upon in the sections that follow. The risk of serious cardiac complications following surgery depends not only on the presence of risk factors, such as those described above, but also varies according to the type of surgery performed. Surgery induces a physiological stress response, with sympatho-humoral activation, increased myocardial oxygen demands and hyper-coagulability. With regard to cardiac risk, surgical interventions fall into one of three categories: low, intermediate or high-risk, according to the risk of myocardial infarction (MI) and cardiac death within 30 days of surgery (Table 2.1). Table 2.1 Risk of MI/cardiac death within 30 days of surgery Cardiopulmonary exercise (CPEX) testing is increasingly regarded as a gold-standard for preoperative exercise testing, yielding considerable data on oxygen uptake and utilization. CPEX testing is cheap and relatively non-invasive, and aims to determine the patient’s anaerobic threshold. Since it evaluates both the cardiovascular and respiratory systems, it is ideal for investigation of the patient with exertional breathlessness. The patient exercises on a bicycle ergometer, with measurement of gas exchange at the mouth together with ECG monitoring. CPEX detects the change from aerobic to partial anaerobic metabolism (Fig. 2.1): at the anaerobic threshold (AT), production of CO2 relative to consumption of O2 increases. An AT of less than 11 ml/min/kg has been associated with a higher perioperative cardiovascular mortality. Part of the physiological stress response to surgery is a catecholamine surge with increased heart rate and myocardial oxygen consumption. In surgical patients with known ischaemic heart disease, Mangano et al5 reported a reduced 2 year mortality after 7 days’ perioperative β-blockade.1 These findings were swiftly incorporated into new guidelines recommending use of β-blockade in patients with overt ischaemic heart disease or with risk factors. Subsequent studies produced more equivocal results and a more cautious approach followed, recommending use of β-blockers in high-risk patients rather than in all patients at risk. Then came the POISE (PeriOperative Ischaemia Study Evaluation) study,6 which measured 30-day mortality and morbidity after oral metoprolol. There was a significant reduction in the number of cardiac events, but the overall mortality rate actually increased, with a significant excess of strokes – possibly because of the excess of patients suffering from hypotension and bradycardia amongst those treated. Close monitoring of blood pressure and heart rate intra- and postoperatively is, however, essential. Two sorts of stent are commonly employed: bare-metal stents have generally been superseded by drug-eluting stents which carry a reduced risk of re-stenosis but a higher risk of stent thrombosis. Drug eluting stents require continuous dual antiplatelet therapy (aspirin + clopidogrel) for at least 12 months after implantation. It is now generally accepted that elective surgery should not take place within 12 months of drug-eluting stent implantation. After 12 months, surgery can proceed, but with at least continuation of aspirin therapy. It is no longer acceptable simply to discontinue all antiplatelet therapy in all patients, and discussion between surgeon, anaesthetist and cardiologist is to be recommended. The recommendations in respect of the timing of non-cardiac surgery after PCI are summarized in Figure 2.2. 1. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977;297(16):845–50. 2. Detsky AS. Cardiac assessment for patients undergoing non cardiac surgery: a multifactorial clinical risk index. Arch Intern Med 1996;146(11):2131–4. 3. Lee TH. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9. 4. Fleisher LA. ACC / AHA 2007 Guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007;50(17):e159–242. 5. Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischaemia Research Group. N Engl J Med 1996;335(23):1713–20. 6. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371:1839–47. Atkinson D, Carter A. Pre-operative assessment for aortic surgery. Current Anaesthesia and Critical Care 2008;19:115–27. Foex P, Sear JW. Challenges of β-blockade in surgical patients. Anesthesiology 2010;113:767–71. Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009;30(22):2769–812. Mild-to-moderate hypoxaemia during the postoperative period is extremely common and may contribute to poor outcome in a variety of areas (Box 2.4). From first principles, adequate tissue oxygenation depends on: Anaesthesia and surgery may disrupt each of these processes. The main factors that contribute to postoperative hypoxaemia are conveniently classified anatomically from respiratory drive onwards, and are summarized in Box 2.5. Increasing the inspired concentration of oxygen provides a higher gradient for diffusion of oxygen from the alveolar gas into the pulmonary capillary blood. Two sorts of device are available – variable and fixed performance (Fig. 2.3). The addition of a reservoir bag increases the amount of inspired oxygen, up to about 80%. The common causes in surgical patients are as listed in Box 2.5 and the clinical manifestations are as described above. In terms of investigations, these should include arterial blood gas analysis and an urgent chest X-ray (CXR). It is important to note that arterial gases do not require to be taken on air for a diagnosis to be made – this is dangerous, and may provoke severe desaturation.
Anaesthesia-related techniques
TECHNIQUES TO ASSESS PERIOPERATIVE RISK
Aims of preoperative assessment in respect of the high-risk patient
 To quantify known disease, and to identify subclinical disease, aiming to intervene and optimize where possible
To quantify known disease, and to identify subclinical disease, aiming to intervene and optimize where possible
 To facilitate informed patient consent: a better appreciation of risk allows patients and clinicians to discuss the risk–benefit ratios of alternative procedures and/or conservative treatment
To facilitate informed patient consent: a better appreciation of risk allows patients and clinicians to discuss the risk–benefit ratios of alternative procedures and/or conservative treatment
 To assist in appropriate allocation of critical care or high-dependency beds
To assist in appropriate allocation of critical care or high-dependency beds
 To assist decision-making in respect of both anaesthesia and surgery: for example, in deciding between open or laparoscopic surgery, or whether to use regional techniques as an adjunct or alternative to general anaesthesia.
To assist decision-making in respect of both anaesthesia and surgery: for example, in deciding between open or laparoscopic surgery, or whether to use regional techniques as an adjunct or alternative to general anaesthesia.
Assess
Cardiac risk indices
A step-by-step approach to risk assessment
Assessing the risk of the surgical procedure
Low risk (<1%)
Intermediate risk (1–5%)
High risk (>5%)
Breast
Abdominal
Aortic and major vascular surgery
Dental
Carotid
Peripheral vascular surgery
Endocrine
Endovascular aneurysm repair
Eye
Head and neck
Gynaecology
Neurosurgery
Plastic/reconstructive
Major orthopaedic
Minor orthopaedic
Renal transplant
Minor urology
Major urology
Action
Tests of functional capacity including cardiopulmonary exercise testing
Aftercare
Pharmacological strategies to reduce risk
β-blockers
Management of antiplatelet therapy
REFERENCES
FURTHER READING
OXYGEN THERAPY
Appraise
Rationale for oxygen therapy
Factors contributing to postoperative hypoxaemia
 diffusion of oxygen across alveolus into the pulmonary capillaries
diffusion of oxygen across alveolus into the pulmonary capillaries
 delivery of arterial blood to the tissues and uptake of oxygen.
delivery of arterial blood to the tissues and uptake of oxygen.
Assess
Assessment and detection of hypoxaemia
 altered mental state (disorientation, confusion, etc.)
altered mental state (disorientation, confusion, etc.)
 dyspnoea or tachypnoea (difficulty completing sentences, use of accessory muscles, etc.)
dyspnoea or tachypnoea (difficulty completing sentences, use of accessory muscles, etc.)
 cyanosis (often difficult to detect clinically)
cyanosis (often difficult to detect clinically)
 cardiovascular: tachycardia/hypertension/arrhythmias
cardiovascular: tachycardia/hypertension/arrhythmias
 vasodilatation (headache/bounding pulses) if accompanying hypercarbia.
vasodilatation (headache/bounding pulses) if accompanying hypercarbia.
Action
Oxygen therapy devices
Variable performance devices
Recognition and management of respiratory failure
 Continuous positive airways pressure (CPAP): delivered via a close-fitting mask, maintaining a positive expiratory pressure of 5-15 cm H2O. It increases FRC and reduces the work of breathing. The increase in intrathoracic pressure reduces cardiac preload and afterload and hence CPAP may be of great benefit in acute heart failure. Gastric distension may be a problem.
Continuous positive airways pressure (CPAP): delivered via a close-fitting mask, maintaining a positive expiratory pressure of 5-15 cm H2O. It increases FRC and reduces the work of breathing. The increase in intrathoracic pressure reduces cardiac preload and afterload and hence CPAP may be of great benefit in acute heart failure. Gastric distension may be a problem.
 Non-invasive ventilation (NIV): this applies a positive inspiratory, as well as expiratory, pressure (e.g. BiPAP), and is particularly useful in the presence of raised PaCO2.
Non-invasive ventilation (NIV): this applies a positive inspiratory, as well as expiratory, pressure (e.g. BiPAP), and is particularly useful in the presence of raised PaCO2.
 Invasive ventilation: requires sedation and endotracheal intubation and may supervene from BiPAP in a patient whose gases are deteriorating or who is becoming exhausted.
Invasive ventilation: requires sedation and endotracheal intubation and may supervene from BiPAP in a patient whose gases are deteriorating or who is becoming exhausted.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree