INTRODUCTION
Human immunodeficiency virus (HIV) types, derived from primate lentiviruses, are the etiologic agents of Acquired Immune Deficiency Syndrome (AIDS). The illness was first described in 1981, and HIV-1 was isolated by the end of 1983. Since then, AIDS has become a worldwide epidemic, expanding in scope and magnitude as HIV infections have affected different populations and geographic regions. Millions are now infected worldwide; once infected, individuals remain infected for life. Within a decade, if left untreated, the vast majority of HIV-infected individuals develop fatal opportunistic infections as a result of HIV-induced deficiencies in the immune system. AIDS is one of the most important public health problems worldwide at the start of the 21st century. The development of highly active antiretroviral therapy (HAART) for chronic suppression of HIV replication and prevention of AIDS has been a major achievement in HIV medicine.
PROPERTIES OF LENTIVIRUSES
Important properties of lentiviruses, members of a genus in the Retroviridae family, are summarized in Table 44-1.
| Virion: Spherical, 80–100 nm in diameter, cylindric core |
| Genome: Single-stranded RNA, linear, positive-sense, 9–10 kb, diploid; genome more complex than that of oncogenic retroviruses, contains up to six additional replication genes |
| Proteins: Envelope glycoprotein undergoes antigenic variation; reverse transcriptase enzyme contained inside virions; protease required for production of infectious virus |
| Envelope: Present |
| Replication: Reverse transcriptase makes DNA copy from genomic RNA; provirus DNA is template for viral RNA. Genetic variability is common. |
| Maturation: Particles bud from plasma membrane |
Outstanding characteristics: Members are nononcogenic and may be cytocidal Infect cells of the immune system Proviruses remain permanently associated with cells Viral expression is restricted in some cells in vivo Cause slowly progressive, chronic diseases Replication is usually species-specific Group includes the causative agents of AIDS |
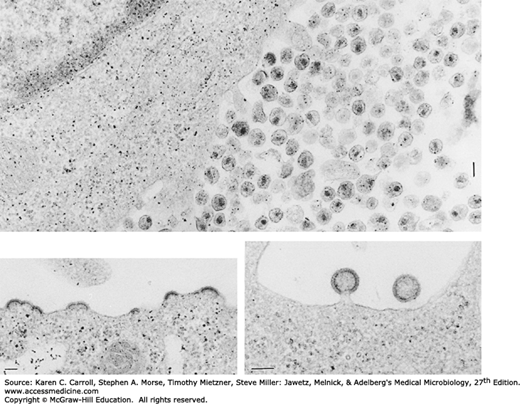
HIV is a retrovirus, a member of the Lentivirus genus, and exhibits many of the physicochemical features typical of the family (see Chapter 43). The unique morphologic characteristic of HIV is a cylindrical nucleoid in the mature virion (Figure 44-1). The diagnostic bar-shaped nucleoid is visible in electron micrographs in those extracellular particles that happen to be sectioned at the appropriate angle.
FIGURE 44-1
Electron micrographs of HIV-infected lymphocytes, showing a large accumulation of freshly produced virus at the cell surface (top, 46,450×, bar = 100 nm); newly formed virus budding from cytoplasmic membrane (lower left, 49,000×, bar = 100 nm); and two virions about to be cast off from cell surface (lower right, 75,140×, bar = 100 nm).
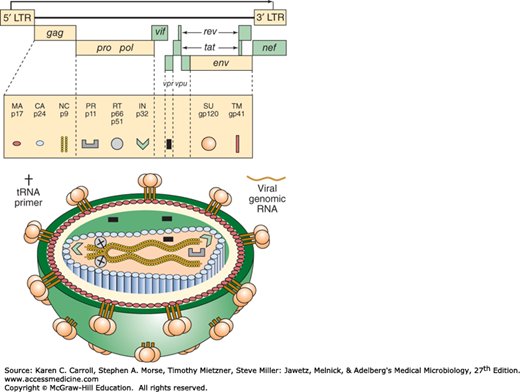
The RNA genome of lentiviruses is more complex than that of transforming retroviruses (Figure 44-2). Lentiviruses contain the four genes required for a replicating retrovirus—gag, pro, pol, and env—and follow the general pattern for retrovirus replication (see Chapter 43). Up to six additional genes regulate viral expression and are important in disease pathogenesis in vivo. Although these auxiliary genes show little sequence homology among lentiviruses, their functions are conserved. (The feline and ungulate viruses encode fewer accessory genes.) One early-phase replication protein, the Tat protein, functions in “transactivation,” whereby a viral gene product is involved in transcriptional activation of other viral genes. Transactivation by HIV is highly efficient and may contribute to the virulent nature of HIV infections. The Rev protein is required for the expression of viral structural proteins. Rev facilitates the export of unspliced viral transcripts from the nucleus; structural proteins are translated from unspliced mRNAs during the late phase of viral replication. The Nef protein increases viral infectivity, facilitates activation of resting T cells, and downregulates expression of CD4 and MHC class I. The nef gene is necessary for simian immunodeficiency virus (SIV) to be pathogenic in monkeys. The Vpr protein increases transport of the viral preintegration complex into the nucleus and also arrests cells in the G2 phase of the cell cycle. The Vpu protein promotes CD4 degradation.
FIGURE 44-2
HIV genome and virion structure. The HIV-1 genome is shown at the top. Viral proteins are synthesized as precursor polyproteins (Gag-Pol [Pr160], Gag [Pr55], and Env [gp160]), which are enzymatically processed to yield mature virion proteins. Gag-Pol and Gag are cleaved by the viral protease PR to produce the indicated smaller proteins. Env is cleaved by a cellular PR, producing SU gp120 and TM gp41. The placements of virion proteins in the virus particle are indicated by symbols (bottom of figure). HIV-2 and SIV lack the vpu gene but contain a vpx gene. (Reproduced from Peterlin BM: Molecular biology of HIV. In Levy JA [editor]. The Viruses. Vol 4: The Retroviridae. Plenum, 1995. Modified there from Luciw PA, Shacklett BL: HIV: Molecular Organization, Pathogenicity and Treatment. Morrow WJW, Haigwood NL [editors]. Elsevier, 1993.)
Cells contain intracellular antiviral inhibitory proteins referred to as restriction factors. One type is APOBEC3G, a cytidine deaminase that inhibits HIV replication. The Vif protein promotes viral infectivity by suppressing the effects of APOBEC3G. Another inhibitory protein is TRIM5α, which binds to incoming retrovirus particles and recruits them to proteasomes before much viral DNA synthesis occurs.
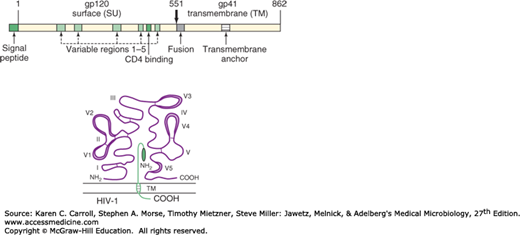
The many different isolates of HIV are not identical but appear to comprise a spectrum of related viruses (see Classification). Heterogeneous populations of viral genomes called quasispecies are found in an infected individual. This heterogeneity reflects high rates of viral replication and the high error rate of the viral reverse transcriptase. The regions of greatest divergence among different isolates are localized to the env gene, which codes for the viral envelope proteins (Figure 44-3). The SU (gp120) product of the env gene contains binding domains responsible for virus attachment to the CD4 molecule and coreceptors, determines lymphocyte and macrophage tropisms, and carries the major antigenic determinants that elicit neutralizing antibodies. The HIV glycoprotein has five variable (V) regions that diverge among isolates, with the V3 region important in neutralization. The TM (gp41) env product contains both a transmembrane domain that anchors the glycoprotein in the viral envelope and a fusion domain that facilitates viral penetration into target cells. The divergence in the envelope of HIV complicates efforts to develop an effective vaccine for AIDS.
FIGURE 44-3
HIV-1 envelope proteins. The gp160 precursor polypeptide is shown at the top. The gp120 subunit is on the outside of the cell, and gp41 is a transmembrane protein. Hypervariable domains in gp120 are designated V1 through V5; the positions of disulfide bonds are shown as connecting lines in the loops. Important regions in the gp41 subunit are the fusion domain at the amino terminal and the transmembrane domain (TM). Amino (NH2) and carboxyl (COOH) terminals are labeled for both subunits. (Reproduced from Peterlin BM: Molecular biology of HIV. In Levy JA [editor]. The Viruses. Vol 4: The Retroviridae. Plenum, 1995. Modified there from Myers G, et al: Human Retroviruses and AIDS 1993: A Compilation and Analysis of Nucleic Acid and Amino Acid Sequences. Theoretical Biology and Biophysics Group T-10, Los Alamos National Library, Los Alamos, New Mexico.)
Lentiviruses are completely exogenous viruses; in contrast to the transforming retroviruses, the lentiviral genome does not contain any conserved cellular genes (see Chapter 43). Individuals become infected by the introduction of virus from outside sources.
Lentiviruses have been isolated from many species (Table 44-2), including more than two dozen different African nonhuman primate species. There are two distinct types of human AIDS viruses: HIV-1 and HIV-2. The two types are distinguished on the basis of genome organization and phylogenetic (evolutionary) relationships with other primate lentiviruses. Sequence divergence between HIV-1 and HIV-2 exceeds 50%.
| Origin of Isolates | Virus | Diseases |
|---|---|---|
| Humans | HIV-1a HIV-2 | Acquired Immune Deficiency Syndrome (AIDS) |
| Nonhuman primatesb | Simian AIDS | |
Chimpanzee Sooty mangabey Macaquesc African green monkey Sykes monkey Mandrill L’Hoest monkeyc Colobus monkey | SIVcpz SIVsm SIVmac SIVagm SIVsyk SIVmnd SIVlhoest SIVcol | |
| Nonprimatesd | ||
Cat Cow Sheep Horse Goat | Feline immunodeficiency virus Bovine immunodeficiency virus Visna/maedi virus Equine infectious anemia virus Caprine arthritis encephalitis virus | Feline AIDS Bovine AIDS Lung, central nervous system disease Anemia Arthritis, encephalitis |
Based on env gene sequences, HIV-1 comprises three distinct virus groups (M, N, and O); the predominant M group contains at least 11 subtypes or “clades” (A–K). Recombinant forms of virus are also found in circulation in humans in different geographic regions. Similarly, eight subtypes of HIV-2 (A–H) have been identified. Within each subtype there is extensive variability. The genetic clades do not seem to correspond to neutralization serotype groups, and there is currently no evidence that subtypes differ in biology or pathogenesis.
Numerous lentivirus isolates have been obtained from nonhuman primate species. The primate lentiviruses fall into six major phylogenetic lineages (Table 44-2). SIV from sooty mangabeys (a type of monkey in West Africa) and HIV-2 are considered to be variants of the same virus, as are chimpanzee isolates and HIV-1. The SIVs from African green monkeys, Sykes monkeys, mandrills, and colobus monkeys represent additional discrete lineages.
The organization of the genomes of primate lentiviruses (human and simian) is very similar. One difference is that HIV-1 and the chimpanzee virus carry a vpu gene, whereas HIV-2 and the SIVsm group have a vpx gene. Other SIV isolates have neither vpu nor vpx genes. The sequences of the gag and pol genes are highly conserved. There is significant divergence among the envelope glycoprotein genes; the sequences of the transmembrane protein portion are more conserved than the external glycoprotein sequences (the protein component exposed on the exterior of the virus particle).
The SIVs appear to be nonpathogenic in their host species of origin (eg, African green monkey, sooty mangabey), species known to be infected in their natural habitats. However, SIVcpz, the precursor of HIV-1, is pathogenic in chimpanzees in the wild, causing AIDS-like pathology and premature death. In contrast, rhesus monkeys are not infected naturally in the wild in Asia but are susceptible to induction of simian AIDS by various SIV isolates. The virus first recovered from captive rhesus monkeys (SIVmac) is the sooty mangabey/HIV-2 strain.
The nonprimate lentiviruses establish persistent infections affecting various animal species. These viruses cause chronic debilitating diseases and sometimes immunodeficiency. The prototype agent, visna virus (also called maedi virus), causes neurologic symptoms or pneumonia in sheep in Iceland. Other viruses cause infectious anemia in horses and arthritis and encephalitis in goats. Feline and bovine lentiviruses may cause an immunodeficiency. Nonprimate lentiviruses are not known to infect any primates, including humans.
HIV in humans originated from cross-species infections by simian viruses in rural Africa, probably due to direct human contact with infected primate blood. Current evidence is that the primate counterparts of HIV-1 and HIV-2 were transmitted to humans on multiple (at least seven) different occasions. Sequence evolution analyses place the introduction of SIVcpz into humans that gave rise to HIV-1 group M at about 1930, although some estimates push the date back to about 1908. Presumably, such transmissions occurred repeatedly over the ages, but particular social, economic, and behavioral changes that occurred in the mid-20th century provided circumstances that allowed these virus infections to expand, become well established in humans, and reach epidemic proportions.
HIV is completely inactivated (≥105 units of infectivity) by treatment for 10 minutes at room temperature with any of the following: 10% household bleach, 50% ethanol, 35% isopropanol, 1% Nonidet P40, 0.5% Lysol, 0.5% paraformaldehyde, or 0.3% hydrogen peroxide. The virus is also inactivated by extremes of pH (pH 1.0, pH 13.0). When HIV is present in clotted or unclotted blood in a needle or syringe, exposure to undiluted bleach for at least 30 seconds is necessary for inactivation.
The virus is not inactivated by 2.5% Tween 20. Although paraformaldehyde inactivates virus free in solution, it is not known if it penetrates tissues sufficiently to inactivate all virus that might be present in cultured cells or tissue specimens.
HIV is readily inactivated in liquids or 10% serum by heating at 56°C for 10 minutes, but dried proteinaceous material affords marked protection. Lyophilized blood products would need to be heated at 68°C for 72 hours to ensure inactivation of contaminating virus.
Insights into the biologic characteristics of lentivirus infections have been gained from experimental infections, including sheep with visna virus (Table 44-2). Natural disease patterns vary among species, but certain common features are recognized.
Viruses are transmitted by exchange of body fluids.
Virus persists indefinitely in infected hosts, although it may be present at very low levels.
Viruses have high mutation rates, and different mutants will be selected under different conditions (host factors, immune responses, tissue types). Infected hosts contain “swarms” of closely related viral genomes, known as quasispecies.
Virus infection progresses slowly through specific stages. Cells in the macrophage lineage play central roles in the infection. Lentiviruses differ from other retroviruses in that they can infect nondividing, terminally differentiated cells. However, those cells must be activated before viral replication ensues and progeny virus is produced. Virus is cell-associated in monocytes and macrophages, but only about one cell per million is infected. Monocytes carry the virus around the body in a form that the immune system cannot recognize, seeding other tissues. Lymphocyte-tropic strains of virus tend to cause highly productive infections, whereas replication of macrophage-tropic virus is restricted.
It may take years for disease to develop. Infected hosts usually make antibodies, but they do not clear the infection, so virus persists lifelong. New antigenic variants periodically arise in infected hosts, with most mutations occurring in envelope glycoproteins. Clinical symptoms may develop at any time, but chronic disease typically manifests after months to years of infection. The exceptions to long incubation periods for lentivirus disease include AIDS in children, infectious anemia in horses, and encephalitis in young goats.
Host factors important in pathogenesis of disease include age (the young are at greater risk), stress (may trigger disease), genetics (certain breeds of animals are more susceptible), and concurrent infections (may exacerbate disease or facilitate virus transmission).
The diseases in horses, sheep, and goats are not complicated by opportunistic secondary infections. Equine infectious anemia virus can be spread among horses by bloodsucking horseflies, the only lentivirus known to be transmitted by an insect vector.
Simian lentiviruses share molecular and biologic characteristics with HIV and cause an AIDS-like disease in rhesus macaques. The SIV model is important for understanding disease pathogenesis and developing vaccine and treatment strategies.
All primate lentiviruses use as a receptor the CD4 molecule, which is expressed on macrophages and T lymphocytes. A second coreceptor in addition to CD4 is necessary for HIV-1 to gain entry to cells. The second receptor is required for fusion of the virus with the cell membrane. The virus first binds to CD4 and then to the coreceptor. These interactions cause conformational changes in the viral envelope, activating the gp41 fusion peptide and triggering membrane fusion. Chemokine receptors serve as HIV-1 second receptors. (Chemokines are soluble factors with chemoattractant and cytokine properties.) CCR5, the receptor for chemokines RANTES, MIP-1α, and MIP-1β, is the predominant coreceptor for macrophage-tropic strains of HIV-1, whereas CXCR4, the receptor for chemokine SDF-1, is the coreceptor for lymphocyte-tropic strains of HIV-1. The chemokine receptors used by HIV for cell entry are found on lymphocytes, macrophages, and thymocytes as well as on neurons and cells in the colon and cervix. Individuals who possess homozygous deletions in CCR5 or produce mutant forms of the protein may be protected from infection by HIV-1; mutations in the CCR5 gene promoter appear to delay disease progression. The requirement for a coreceptor for HIV fusion with cells provided new targets for antiviral therapeutic strategies, with the first HIV entry inhibitor licensed in the United States in 2003.
Another molecule, integrin α-4 β-7, appears to function as a receptor for HIV in the gut. A dendritic cell-specific lectin, DC-SIGN, appears to bind HIV-1 but not to mediate cell entry. Rather, it may facilitate transport of HIV by dendritic cells to lymphoid organs and enhance infection of T cells.
HIV INFECTIONS IN HUMANS
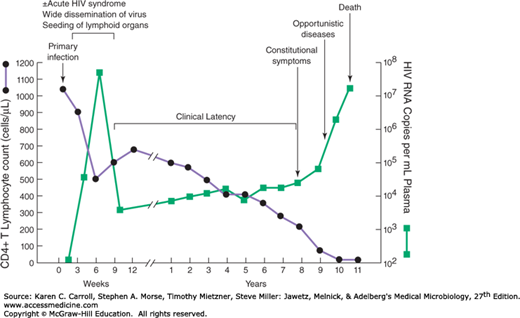
The typical course of untreated HIV infection spans about a decade (Figure 44-4). Stages include the primary infection, dissemination of virus to lymphoid organs, clinical latency, elevated HIV expression, clinical disease, and death. The duration between primary infection and progression to clinical disease averages about 10 years. In untreated cases, death usually occurs within 2 years after the onset of clinical symptoms.
FIGURE 44-4
Typical course of untreated HIV infection. During the early period after primary infection, there is widespread dissemination of virus and a sharp decrease in the number of CD4 T cells in peripheral blood. An immune response to HIV ensues, with a decrease in detectable viremia followed by a prolonged period of clinical latency. Sensitive assays for viral RNA show that virus is present in the plasma at all times. The CD4 T-cell count continues to decrease during the following years until it reaches a critical level below which there is a substantial risk of opportunistic diseases. (Reproduced with permission from Fauci AS, Lane HC: Human immunodeficiency virus disease: AIDS and related disorders. In Longo DL, Fauci AS, Kasper DL, et al (editors). Harrison’s Principles of Internal Medicine, 18th ed. McGraw-Hill, 2012. © The McGraw-Hill Companies, Inc.)
Following primary infection, there is a 4- to 11-day period between mucosal infection and initial viremia; the viremia is detectable for about 8–12 weeks. Virus is widely disseminated throughout the body during this time, and the lymphoid organs become seeded. An acute mononucleosis-like syndrome develops in many patients (50–75%) 3–6 weeks after primary infection. There is a significant drop in numbers of circulating CD4 T cells at this early time. An immune response to HIV occurs 1 week to 3 months after infection, plasma viremia drops, and levels of CD4 cells rebound. However, the immune response is unable to clear the infection completely, and HIV-infected cells persist in the lymph nodes.
This period of clinical latency may last for 10 years or more. During this time, there is a high level of ongoing viral replication. It is estimated that 10 billion HIV particles are produced and destroyed each day. The half-life of the virus in plasma is about 6 hours, and the virus life cycle (from the time of infection of a cell to the production of new progeny that infect the next cell) averages 2.6 days. CD4 T lymphocytes, major targets responsible for virus production, appear to have similar high turnover rates. Once productively infected, the half-life of a CD4 lymphocyte is about 1.6 days.
Viral diversity studies have shown that in most cases of sexual transmission a single HIV variant establishes a new infection. Early in infection, viral sequences are quite homogeneous, but because of rapid viral proliferation and the inherent error rate of the HIV reverse transcriptase, quasispecies of virus accumulate. It is estimated that every nucleotide of the HIV genome probably mutates on a daily basis.
Eventually, the patient develops constitutional symptoms and clinically apparent disease, such as opportunistic infections or neoplasms. Higher levels of virus are readily detectable in the plasma during the advanced stages of infection. HIV found in patients with late-stage disease is usually much more virulent and cytopathic than the strains of virus found early in infection. Often, a shift from monocyte-tropic or macrophage-tropic (M-tropic) strains of HIV-1 to lymphocyte-tropic (T-tropic) variants accompanies progression to AIDS.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree